17 January 2023: Original Paper
Non-Cryopreserved Peripheral Blood Stem Cell Graft for Autologous Hematopoietic Stem Cell Transplantation in Multiple Myeloma and Lymphoma Patients
Panarat Noiperm1ABCDEF, Jakrawadee Julamanee1ABCDEFG*, Pongtep Viboonjuntra1ADE, Arnuparp Lekhakula1ABCDEDOI: 10.12659/AOT.938595
Ann Transplant 2023; 28:e938595
Abstract
BACKGROUND: Hematopoietic stem cell transplantation (HSCT) using cryopreserved grafts is time-consuming, expensive treatment, and may associated with dimethyl sulfoxide (DMSO) toxicity. Here, we assess the clinical utility and safety of non-cryopreserved peripheral blood stem cell graft in autologous HSCT.
MATERIAL AND METHODS: Medical data of multiple myeloma or lymphoma patients who underwent autologous non-cryopreserved HSCT were reviewed.
RESULTS: A total of 58 patients (40 myeloma and 18 lymphoma) were reviewed. The median myeloma and lymphoma CD34⁺ cell doses were 7.59 and 6.9 million/kg, respectively, with good viability after storage. The median times in neutrophil and platelet engraftment were 9 and 13 days and 11 and 14 days in myeloma and lymphoma, respectively. Only 5 patients in this cohort developed serious post-transplant complications. After transplantation, the cumulative incidence of relapse at 5 years was 34.4% in myeloma versus 19.1% in lymphoma patients. Notably, the mortality incidence rate rapidly increased within the first year and reached a plateau after 4 years, with cumulative incidence of 5.9% and 30.9% in myeloma and lymphoma, respectively. With a median follow-up time of 60 months, the median progression-free survival (PFS) and overall survival (OS) for lymphoma patients was 123.8 and 130 months, respectively. For the myeloma group, the median follow-up time was 38.6 months, the median PFS was 99.5 months, and OS was 157 months.
CONCLUSIONS: Non-cryopreserved HSCT is effective and safe. The long-term survival outcomes could be achieved by the shortening the duration of neutrophil and platelet engraftments and the complication rates are acceptable.
Keywords: autografts, Bone Marrow Transplantation, Cryopreservation, Lymphoma, Multiple Myeloma, Humans, peripheral blood stem cells, Neoplasm Recurrence, Local, Hematopoietic Stem Cell Transplantation, Peripheral Blood Stem Cell Transplantation, Transplantation, Autologous
Background
Hematopoietic stem cell transplantation (HSCT) is a curative treatment option with significantly longer disease-free survival (DFS) and overall survival (OS) compared to other treatment modalities for various hematologic malignancies [1]. HSCT procedures include stem cell collection, cryopreserved storage, conditioning chemotherapy, and stem cell reinfusion. Stem cells can be harvested directly from bone marrow aspirate, peripheral blood after mobilization, and umbilical cord blood. The cryopreservation technique is performed by adding dimethyl sulfoxide and Anticoagulant Citrate Dextrose Solution, Solution A to the stem cells to maintain viability until reinfusion [2]. However, cryopreserved HSCT is laborious, expensive, and involves significant cryo-injury to the cells via dimethyl sulfoxide toxicity. Recently, several investigators demonstrated the successful treatment of non-cryopreserved HSCT in resource-limited centers [3–9]. Moreover, non-cryopreserved HSCT is associated with shorter hospital stay and lower febrile neutropenia and mucositis rates [8]. In this study, we assessed the clinical utility and safety of non-cryopreserved unmanipulated peripheral blood stem cells (PBSCs) in adult patients with multiple myeloma or malignant lymphoma who underwent autologous HSCT in Songklanagarind Hospital from 1996 to 2021.
Material and Methods
STUDY DESIGN:
The study protocol (REC. 64-188-14-1) was approved by the Human Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University. This retrospective cohort study was conducted in Songklanagarind Hospital, a tertiary university hospital located in southern Thailand, in accordance with the Declaration of Helsinki. We reviewed the records of myeloma or lymphoma patients aged 18 years or older who underwent non-cryopreserved HSCT in Songklanagarind Hospital from January 1996 to March 2021. Patients with insufficient clinical data for review were excluded.
Before transplantation, all patients were assessed for clinical and disease status, including age, sex, Eastern Cooperative Oncology Group performance status, laboratory test results, organ function, diagnosis, stage, treatment options, and disease response. Hematopoietic cell transplant comorbidity index scores were calculated to assess the transplantation comorbidity risk [10].
MOBILIZATION TECHNIQUE AND PBSC COLLECTION:
All autologous stem cell transplantation (ASCT) procedures were performed using PBSCs. A central venous catheter (Hickman line) was placed via the internal jugular or subclavian vein in all patients 1 week before transplantation. The PBSCs were mobilized using a combination of chemotherapy, cyclophosphamide 4 gm/m2 with or without etoposide 300 mg/m2, or ICE regimen (etoposide 100 mg/m2 day 1–3, ifosfamide 5 gm/m2 day 2, and carboplatin 5 AUC day 2) followed by G-CSF 10 μg/kg/d daily subcutaneous injection for 4–5 consecutive days or G-CSF alone. Peripheral blood CD34+ cells were measured daily each morning by flow cytometer after the end of the nadir phase. PBSC mobilization was performed when the circulating CD34+ cell count was greater than 10/μl using a Spectra Optia® separator (Cobe Laboratories, Inc., Lakewood, CO, USA) or a COM.TEC® separator (Fresenius Kabi, Germany). Apheresis was performed daily until reaching the goal of ≥2×106 peripheral blood CD34+ cells per kilogram. The PBSC samples were stained with 7-amino-actinomycin D and analyzed by flow cytometer to assess cell viability before storage. The final PBSC products were then stored at 4°C in a blood bank refrigerator until the day of stem cell infusion. In the early phase, aliquots of stem cell products were assessed daily for viability by trypan blue dye exclusion test (Figure 1).
PRE-TRANSPLANT CONDITIONING AND PBSC TRANSPLANTATION:
High-dose chemotherapy was administered immediately after complete stem cell harvesting. The conditioning regimen for multiple myeloma patients was melphalan 200 mg/m2 or 140 mg/m2, depending on patient performance and renal function status. In malignant lymphoma, various conditioning regimens were administered, mainly BEAM (BCNU 300 mg/m2 day -6, etoposide 800 mg/m2 day -5 to -2, cytarabine 1600 mg/m2 day -5 to -2, and melphalan 140 mg/m2 day -1) and BEAC (BCNU 300 mg/m2 day -7, etoposide 800 mg/m2 day -6 to -3, cytarabine 800 mg/m2 day -6 to -3, and cyclophosphamide 140 mg/kg day -6 to -3). Stem cells were infused during a period of 24 h on day 0 after completion of the conditioning regimen. At the time of reinfusion, all apheresis stem cells were assessed for viability by the trypan blue staining method. Aliquots of apheresis products were also tested for bacterial contamination before storage and at the time of reinfusion. Patients received a daily injection of G-CSF 5–10 g/kg/d starting 3–5 days after stem cell infusion and continued until neutrophil engraftment.
After transplantation, all patients were hospitalized in an isolated high positive pressure room to prevent airborne particles traveling into the room, which would contaminant the surrounding environment. During transplantation, patients received antiviral, antifungal, and antibacterial prophylaxis on the day of starting the conditioning regimen until the day of neutrophil engraftment.
DEFINITIONS AND EVALUATION:
Neutrophil engraftment was defined as an absolute neutrophil count ≥500/mm3 for 3 consecutive days. Platelet engraftment was defined as a platelet count ≥20 000/m3 for 3 consecutive days and no transfusions for 7 days. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.
STATISTICAL ANALYSIS:
Study data were analyzed using SPSS version 28.0 (IBM, SPSS, Inc., Chicago, IL, USA) and Stata version 17.0 (StataCorp LLC, TX, USA). Before analysis, subjects with incomplete data were removed from the dataset. Continuous data are presented as mean or median with range. Categorical data are presented as number and percentage for baseline characteristics, transplant parameters, and peri-transplant outcomes. The cumulative incidence function (CIF) with competing risk regression analyses of relapse and mortality after transplantation were calculated using the Fine-Grey model [11]. OS was calculated as the time interval from the date of transplantation to the date of death or last follow-up, and progression-free survival (PFS) was calculated as the time interval from the date of transplantation to the date of relapse or last follow-up. Survival times were analyzed by the Kaplan-Meier method and log-rank test. Multivariate analyses for survival were performed using a Cox regression model. Differences were considered statistically significant when the P values were <0.05.
Results
VIABILITY OF NON-CRYOPRESERVED STEM CELL GRAFT:
Non-cryopreserved ASCT was initiated in our center in 1995 using PBSC graft. In the early phase, the viability of each stem cell product was assessed daily using the trypan blue exclusion test after storage in a 4°C conventional blood bank refrigerator to confirm the feasibility of non-cryopreserved HSCT. Of the preliminary 37 patients, the stem cell products were stored for a median of 6 days (range 4–7 days) with a median viability of 92% (range 82–98%). The viability results of the stored stem cell products are shown in Figure 1. The preliminary results indicated that the stem cell products had acceptable viability in a non-cryopreserved liquid storage environment for up to 7 days and feasible to use in long schedules of high-dose chemotherapy, especially in lymphoma patients.
DEMOGRAPHIC DATA:
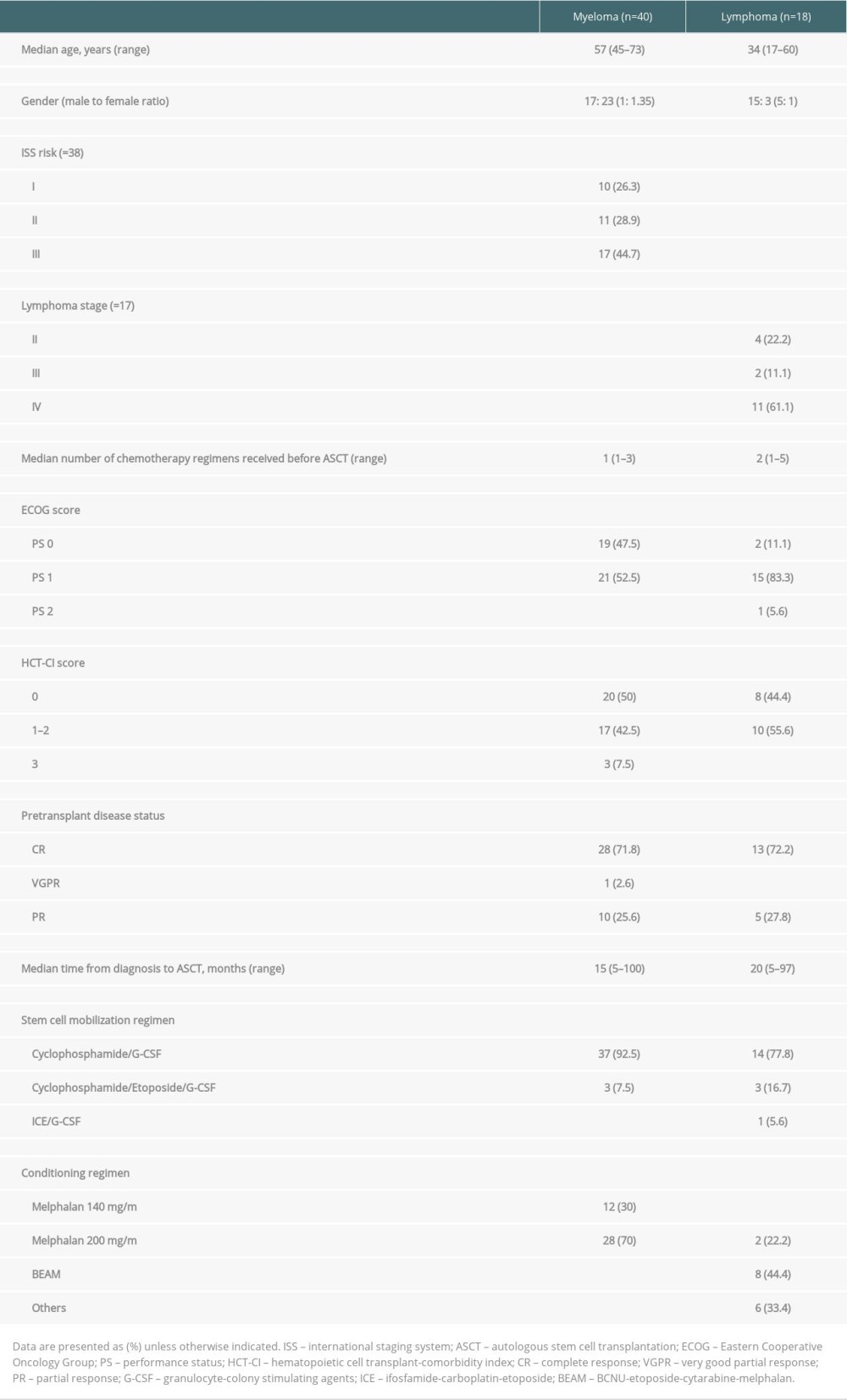
Of a total of 81 patients who received ASCT during January 1996 to March 2021, 58 patients had adequate data and were reviewed in this cohort. Eighteen and 40 patients were diagnosed as having lymphoma and multiple myeloma, respectively. Nineteen patients were excluded due to insufficient data to review. The baseline patient characteristics are shown in Table 1. The median age of the myeloma patients was older than in the lymphoma group. Compared to myeloma patients, the lymphoma patients were predominantly male. In both subgroups, the majority of the patients were diagnosed as advanced stage. All patients received pre-transplant induction chemotherapy and were mostly in remission prior to transplantation. The median times from diagnosis to ASCT were 15 and 20 months in the myeloma and lymphoma groups, respectively. High-dose cyclophosphamide following by G-CSF was frequently used in the mobilization regimen in both groups. High-dose melphalan was used as the conditioning regimen for all myeloma patients, while the BEAM regimen was mainly used in lymphoma patients.
TRANSPLANT PARAMETERS AND OUTCOMES:
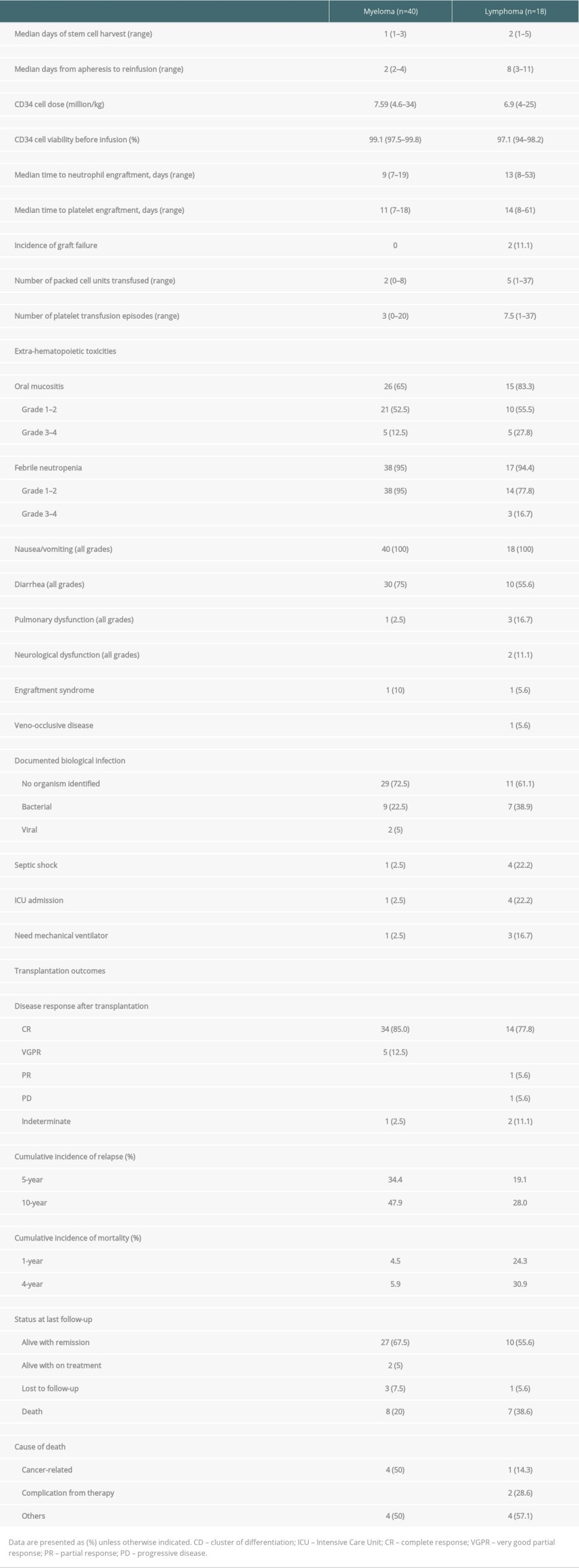
The transplant parameters are summarized in Table 2. The median number of days of stem cell harvest was 1 day (range 1–3) and 2 days (range 1–5) in the myeloma and lymphoma groups, respectively. The median CD34+ cells in myeloma and lymphoma were 7.59 million/kg (range 4.6–34) and 6.9 million/kg (range 4–25), respectively. The pre-infusion stem cell viability rates were above 97% in both groups. There was no incidence of bacterial contamination in stem cell products. The median time from apheresis to reinfusion was 2 days in myeloma patients and 8 days in lymphoma patients. No adverse events occurred between stem cell collection and conditioning in which the stem cell transplantation was interrupted or delayed in this cohort. Engraftment failure was observed in 2 lymphoma patients; both patients were diagnosed with stage IV mantle cell lymphoma with bone marrow involvement and peripheral T-cell lymphoma, respectively. The adequate stem cell dosages were reinfused after the conditioning regimen (total CD34+ cells were 5.6 and 25.0 million/kg, respectively, after mobilization). Unfortunately, they developed septic shock related to blood stream and gastrointestinal tract infections and died shortly after transfer to the intensive care unit. The median times of neutrophil engraftment and platelet engraftment in the myeloma and lymphoma patients were 9 days (range 7–19) and 13 days (range 8–53) and 11 days (range 7–18) and 14 days (range 8–61), respectively.
At the peri-transplant period, the median number of packed cells and platelet transfusions were 5 and 7.5 units in the lymphoma group, which were higher than in the myeloma group. Extra-hematopoietic toxicities occurred in 65% of the myeloma patients and 83% of the lymphoma patients, which manifested as grade 1–2 mucositis in most patients during transplantation. All patients experienced some nausea or vomiting, while half of the patients developed diarrhea. In both groups, febrile neutropenia occurred in more than 95% of patients and most were grade 1–2. Although no organism was identified in the majority of cases, bacterial infection was identified in one-third of the patients. Severe complications that included septic shock and respiratory failure that required intensive care unit and mechanical ventilator support in this cohort were observed in 5 patients (1 myeloma and 4 lymphoma). Engraftment syndrome was observed in only 2 patients: 1 in each group. Veno-occlusive disease was diagnosed in 1 lymphoma patient. All patients with complications were clinically diagnosed without pathological confirmation.
TRANSPLANTATION OUTCOMES:
The transplant outcomes are summarized in Table 2. Eighty-five percent of the myeloma patients were in complete remission and 12.5% were in very good partial remission after transplantation. In the lymphoma patients, 77% were in complete remission and 5.6% were in partial remission. After transplantation, both diseases gradually relapsed, with the 5-year CIF of 34.4% in myeloma patients and 19.1% in lymphoma patients. Notably, the mortality rate rapidly increased within the first year and reached a plateau after 4 years in the lymphoma group, with a 4-year CIF of 30.9% compared to 5.9% in the myeloma group. The CIF with competing risk regression analyses of relapse and mortality after transplantation are shown in Figure 2.
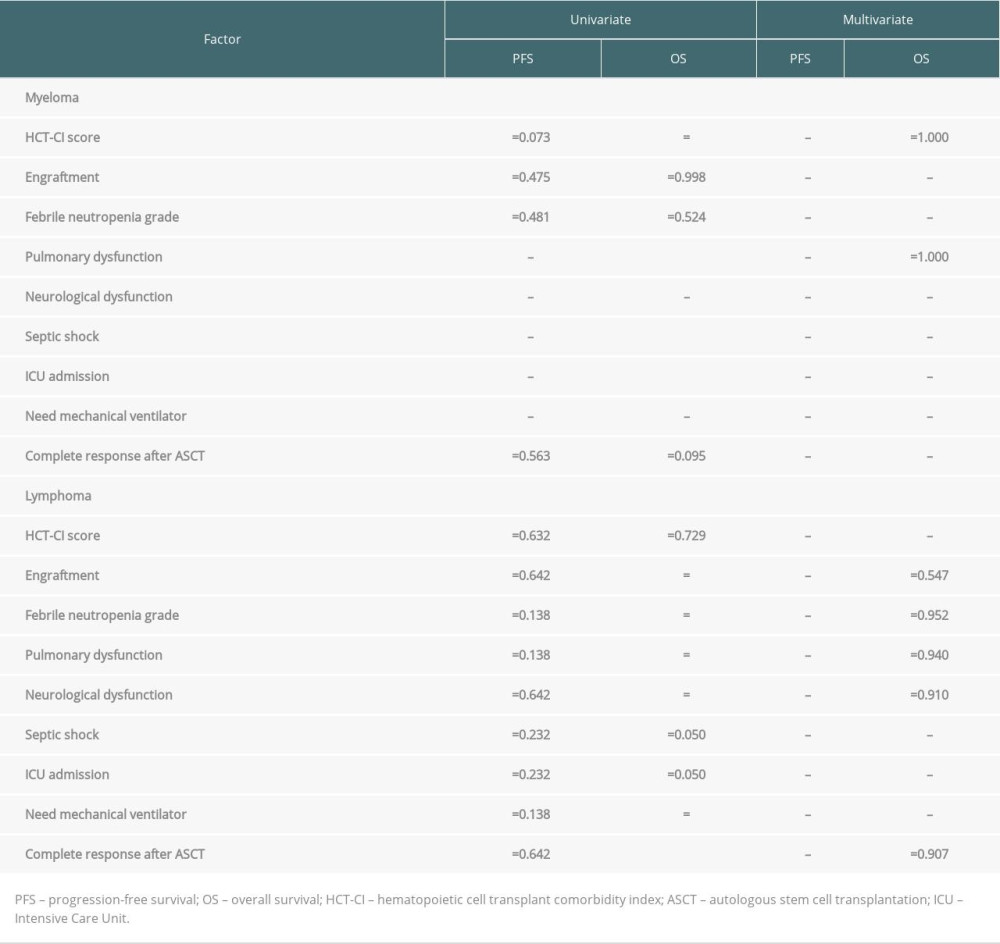
With the median follow-up time of 60 months and 63 months (range 7.2–146.2), the median PFS and OS for lymphoma patients were 123.8 and 130 months, respectively. For the myeloma group, the median follow-up time was 38.6 months (range 12.9–144.3) and 48.6 months (range 12.91–157.04), the median PFS was 99.5 months, and OS was 157 months. The survival rates of the myeloma and lymphoma patients are shown in Figure 3. At the time of the analysis, the myeloma and lymphoma patients were alive with remission in 67.5% and 55.6%, respectively. Cancer-related death was observed in half of the myeloma patients, whereas one-fourth of the lymphoma patients died from therapy-related complications. We analyzed the effect of various factors on patient survival (Table 3). In myeloma, hematopoietic cell transplant comorbidity index score, pulmonary complication during ASCT, septic shock, and intensive care unit admission significantly predicted OS; however, none of them were significant in multivariate results. In the lymphoma group, engraftment, febrile neutropenic grade, pulmonary dysfunction, neurological dysfunction, need for mechanical ventilation, and complete response after ASCT were statistically significant to predict OS in univariate analysis, but none of them were significant in multivariate analysis. In addition, none of the factors were associated with PFS in myeloma and lymphoma patients in both univariate and multivariate analyses.
Discussion
The present study demonstrated the feasibility of non-cryopreserved stem cell graft for autologous HSCT in both multiple myeloma and malignant lymphoma. Various studies have reported success in non-cryopreserved stem cell graft in autologous HSCT. However, only a study by Kardduss-Urueta et al demonstrated a viability of 88% in stem cell grafts after storage before reinfusion, which is essential for lymphoma patients who need long-term conditioning regimens [3–9]. The present study supports the hypothesis that autograft preparations can be safely and effectively kept at 4°C, with high viability rates (99.1% and 97.1% for myeloma and lymphoma, respectively) up to 7 days before reinfusion.
In our center, PBSCs were mobilized mainly with cyclophosphamide and G-CSF, which was different from other centers, where G-CSF alone was frequently used for mobilization. This combination regimen demonstrated higher median CD34+ cell doses (7.59 million/kg and 6.9 million/kg in myeloma and lymphoma, respectively) compared to other centers, which were in the range of 2.9–5.7 million/kg [3,4,6–9]. Moreover, stem cell apheresis was successful at a median of only 1 or 2 days, which is advantageous for lymphoma patients, whose stem cell products need to be kept for over 1 week before reinfusion. Several studies reported that the duration of neutrophil engraftment was shorter in non-cryopreserved HSCT [4,5] In the present study, we also observed early neutrophil and platelet engraftment with lower rates of graft failure, which was possibly due to the high stem cell dose and high viability. We also observed earlier neutrophil and platelet engraftment in the myeloma patients compared to the lymphoma group, which resulted in fewer packed cell and platelet transfusions. In this cohort, 2 lymphoma cases had failure to engraft due to severe infection that led to poor outcomes. Compared to previous studies, the myeloma patients in this study had high pre-transplant tumor burden, as evidenced by the high proportion of international staging system stage III, while the disease response after transplantation demonstrated greater improvement of disease, as observed by the high proportion of very good partial response and complete response in 97.5% [4–6,9]. In the current study, improvement of disease in the lymphoma group was only an increase of 5% in the complete response rate.
We observed a lower overall incidence of oral mucositis than in previous studies, which predominantly affected lymphoma patients due to the high-intensity conditioning regimen [4,12]. Previously, serious complications, such as respiratory failure or neurological dysfunction, were reported in about 2% of myeloma patients, which was similar to our study [9]. While none of the myeloma patients in our cohort developed grade 3–4 febrile neutropenia, septic shock and respiratory failure were observed in 20% of the lymphoma patients, which was possibly related to the high-intensity conditioning regimen and chemotherapy regimens received before ASCT.
After transplantation, the cumulative incidence of relapse gradually increased over time and finally reached a plateau at around 7 years, and we observed a tendency of higher relapse rate in myeloma compared to lymphoma patients. In terms of survival, the mortality rate rapidly increased within the first year and reached a plateau 4 years after transplantation, especially in the lymphoma group. To the best of our knowledge, limited information is available on long-term survival outcomes in non-cryopreserved HSCT. However, the current study demonstrated long-term PFS and OS in both myeloma and lymphoma patients compared to previous reports, while none of the studied factors significantly predicted survival [3,4,9]. Our study has certain limitations – we enrolled a small number of patients and did not compare the outcomes between cryopreserved and non-cryopreserved stem cell transplantation. Therefore, a randomized control study including more patients or multicenter studies are required to confirm our results.
Conclusions
Non-cryopreserved autologous HSCT is feasible, effective, and safe to perform in multiple myeloma and lymphoma patients. Using this simple non-cryopreserved procedure, many viable stem cells with a short duration of hematological reconstitution can be obtained, which promotes non-cryopreserved HSCT as an alternative and useful procedure, particularly in areas with logistic constraints and limited resources.
Figures
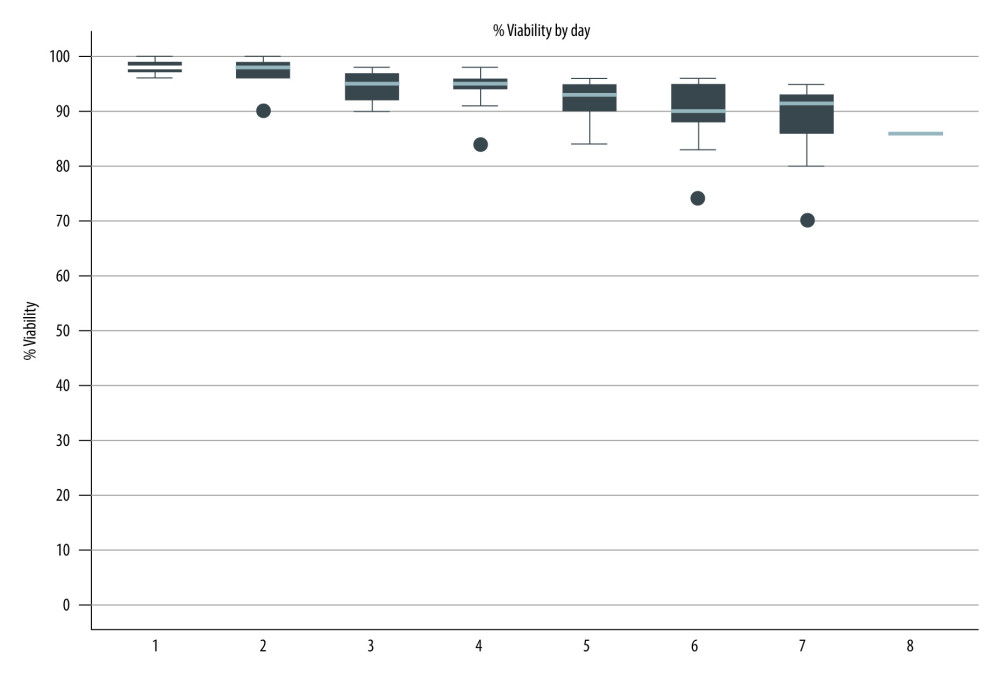 Figure 1. Viability of stored stem cell products. (Stata software, version 17.0, StataCorp LLC, TX, USA).
Figure 1. Viability of stored stem cell products. (Stata software, version 17.0, StataCorp LLC, TX, USA). 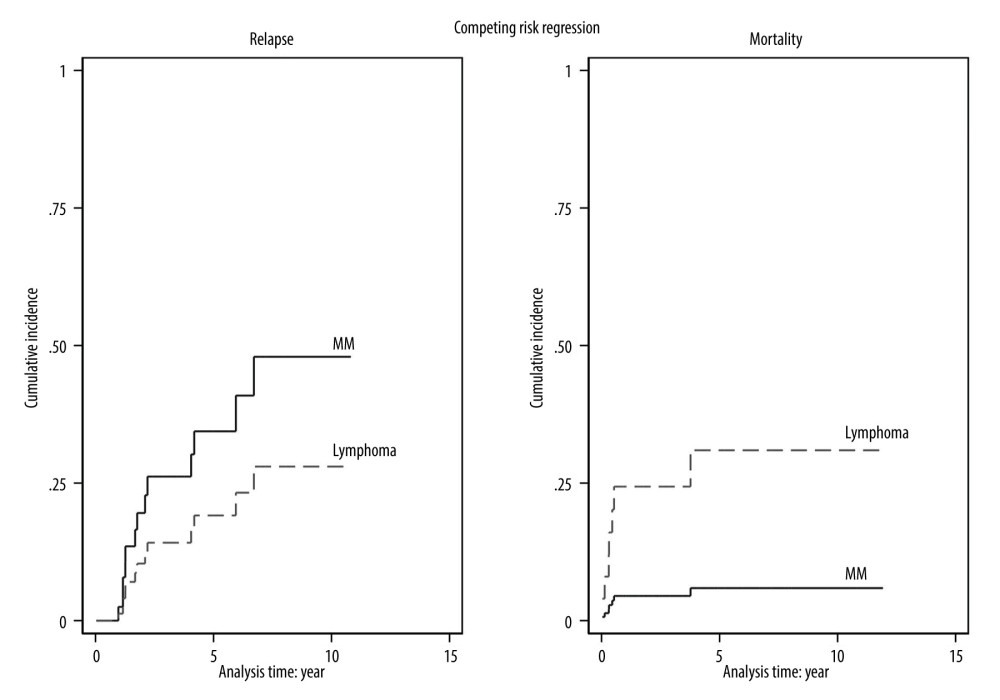 Figure 2. The cumulative incidence function with competing risk regression analyses of relapse and mortality after transplantation. (Stata software, version 17.0, StataCorp LLC, TX, USA).
Figure 2. The cumulative incidence function with competing risk regression analyses of relapse and mortality after transplantation. (Stata software, version 17.0, StataCorp LLC, TX, USA). 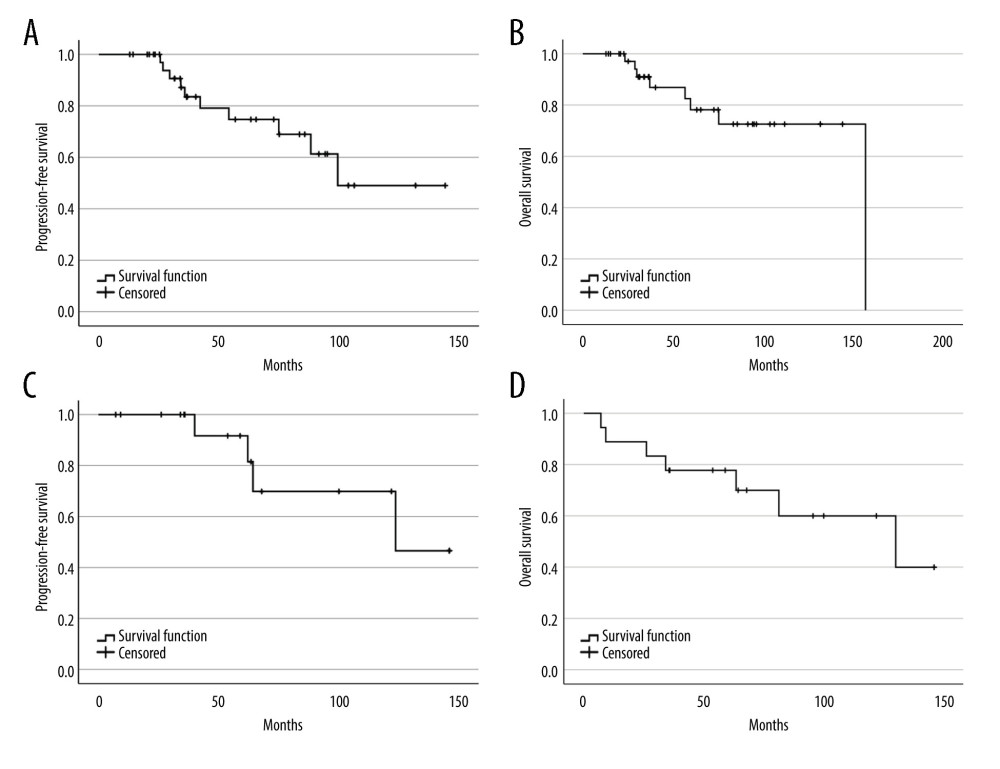 Figure 3. Progression-free survival and overall survival of myeloma (A, B) and lymphoma (C, D). (SPSS software, version 28.0, IBM, SPSS, Inc., Chicago, IL, USA).
Figure 3. Progression-free survival and overall survival of myeloma (A, B) and lymphoma (C, D). (SPSS software, version 28.0, IBM, SPSS, Inc., Chicago, IL, USA). References
1. Mahajan S, Tandon N, Kumar S, The evolution of stem-cell transplantation in multiple myeloma: Ther Adv Hematol, 2018; 9(5); 123-33
2. Wuchter P, Processing, cryopreserving and controlling the quality of HSCs: The EBMT handbook, hematopoietic stem cell transplantation and cellular therapies, 2019; Chapter 17; 127-30, Switzerland, Springer Nature
3. Bekadja MA, Boumendil A, Blaise D, Non-cryopreserved hematopoietic stem cells in autograft patients with lymphoma: A matched-pair analysis comparing a single center experience with the use of cryopreserved stem cells reported to the European Society for Blood and Marrow Transplantation registry: Cytotherapy, 2021; 23(6); 483-87
4. Piriyakhuntorn P, Tantiworawit A, Rattanathammethee T, Outcomes of non-cryopreserved versus cryopreserved peripheral blood stem cells for autologous stem cell transplantation in multiple myeloma: Ann Transplant, 2020; 25; e927084
5. Jennane S, Hasnaoui N, Mahtat EM, Non-cryopreserved peripheral blood stem cells autologous transplantation in multiple myeloma: Bicentric study: Transfus Clin Biol, 2020; 27(3); 152-56
6. Kulkarni U, Devasia AJ, Korula A, Use of non-cryopreserved peripheral blood stem cells is associated with adequate engraftment in patients with multiple myeloma undergoing an autologous transplant: Biol Blood Marrow Transplant, 2018; 24(12); e31-e35
7. Kardduss-Urueta A, Gale RP, Gutierrez-Aguirre CH, Freezing the graft is not necessary for autotransplants for plasma cell myeloma and lymphomas: Bone Marrow Transplant, 2018; 53(4); 457-60
8. Sarmiento M, Ramírez P, Parody R, Advantages of non-cryopreserved autologous hematopoietic stem cell transplantation against a cryopreserved strategy: Bone Marrow Transplant, 2018; 53(8); 960-66
9. Kayal S, Sharma A, Iqbal S, High-dose chemotherapy and autologous stem cell transplantation in multiple myeloma: A single institution experience at All India Institute of Medical Sciences, New Delhi, using non-cryopreserved peripheral blood stem cells: Clin Lymphoma Myeloma Leuk, 2014; 14(2); 140-47
10. Sorror ML, Maris MB, Storb R, Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT: Blood, 2005; 106(8); 2912-29
11. Lau B, Cole SR, Gange SJ, Competing risk regression models for epidemiologic data: Am J Epidemiol, 2009; 170(2); 244-56
12. Naithani R, Dayal N, Pathak S, Rai R, Hematopoietic stem cell transplantation using non-cryopreserved peripheral blood stem cells graft is effective in multiple myeloma and lymphoma: Bone Marrow Transplant, 2018; 53(9); 1198-200
13. Noiperm P, Julamanee J, Viboonjuntra P, Lekhakula A, Non-cryopreserved peripheral blood stem cell graft for autologous hematopoietic stem cell transplantation in multiple myeloma and lymphoma: Bone Marrow Transplant, 2022(P254); 100-416
Figures
 Figure 1. Viability of stored stem cell products. (Stata software, version 17.0, StataCorp LLC, TX, USA).
Figure 1. Viability of stored stem cell products. (Stata software, version 17.0, StataCorp LLC, TX, USA). Figure 2. The cumulative incidence function with competing risk regression analyses of relapse and mortality after transplantation. (Stata software, version 17.0, StataCorp LLC, TX, USA).
Figure 2. The cumulative incidence function with competing risk regression analyses of relapse and mortality after transplantation. (Stata software, version 17.0, StataCorp LLC, TX, USA). Figure 3. Progression-free survival and overall survival of myeloma (A, B) and lymphoma (C, D). (SPSS software, version 28.0, IBM, SPSS, Inc., Chicago, IL, USA).
Figure 3. Progression-free survival and overall survival of myeloma (A, B) and lymphoma (C, D). (SPSS software, version 28.0, IBM, SPSS, Inc., Chicago, IL, USA). In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860