20 June 2023: Original Paper
Survival Analysis of Transplant-Associated Thrombotic Microangiopathy Under Different Diagnostic Criteria and Efficacy of Plasma Exchange
Yifan XuDOI: 10.12659/AOT.939890
Ann Transplant 2023; 28:e939890
Abstract
BACKGROUND: Transplant-associated thrombotic microangiopathy (TA-TMA) is a serious complication of hematopoietic stem cell transplantation (HSCT). The efficacy and survival of plasma exchange (PE) for TA-TM have not been fully clarified. In addition, there is a lack of consensus on diagnostic criteria for TA-TMA.
MATERIAL AND METHODS: We retrospectively analyzed 32 patients diagnosed with TA-TMA by different diagnostic criteria from January 2018 to February 2022 at the First Medical Center of the PLA General Hospital.
RESULTS: (1) The patients with TA-TMA treated with PE in this study had a remission rate of 42.8%, a 100-day OS of 47.6%, and a 6-month OS of 38.1%. The only factor affecting the response to PE treatment was the number of PE sessions (P = 0.047). (2) III-IV aGVHD prior to TA-TMA diagnosis (P = 0.002), renal or neurological dysfunction (P = 0.021), and the time to onset of TA-TMA (P = 0.002) were independent risk factors for overall survival with TA- TMA. (3) Probable TA-TMA had the highest survival rate, but the Jodele criteria are expected to diagnose earlier and provide the greatest benefit to patients.
CONCLUSIONS: PE is an effective treatment for TA-TMA especially in cases where complement blockers are not available. In addition, probable TA-TMA improved prognostic survival through early detection of patients with TA-TMA. There is a need for further large prospective trials to identify the population more suitable for PE treatment of TA-TMA and more valid diagnostic criteria.
Keywords: Hematopoietic Stem Cell Transplantation, Plasma Exchange, Purpura, Thrombotic Thrombocytopenic, Humans, Retrospective Studies, Prospective Studies, Thrombotic Microangiopathies, Survival Analysis
Background
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is effective for various hematologic disorders. The number of HSCT cases has progressively increased in recent years with advances in transplantation technology. Transplant-associated thrombotic microangiopathy (TA-TMA) is a serious complication of HSCT, with studies reporting a morbidity rate of 2.3% to 39% and a mortality rate of more than 80% [1]. The morbidity and mortality rates are highly variable due to inconsistent diagnostic criteria and the variety of treatments used. TA-TMA involves multiple organs, including the kidneys, nerves, gastrointestinal tract, lungs, and heart, and is associated with microangiopathic hemolysis and micro-thrombosis [2,3], but its pathogenesis remains unclear. The current treatment of TA-TMA includes supportive therapy such as reduction/discontinuation of calcineurin inhibitor (CNI)/mTOR inhibitors, control of hypertension, and treatment of comorbidities that can trigger TA-TMA, such as GVHD and infection. Other treatments include plasma exchange (PE), complement blocking therapy, and defibrotic therapy [4–7]. PE can improve the acute symptoms in the short term, but the long-term prognostic impact has not been clarified. Historically, PE has been used as an important treatment for TA-TMA due to the good efficacy and prognosis survival of PE in the treatment of thrombocytopenic purpura (TTP) [8]. However, a low survival rate (21%) was reported in 121 patients with TA-TMA routinely treated with PE [9]. In another single-center study, 66 patients developed TA-TMA after HSCT while receiving tacrolimus, and 60% of the 63 patients treated with PE developed a response. The 6-month survival rate was 0% for those who did not respond and 50% for those who did respond [10]. All suggested no significant improvement in the prognostic survival of TA-TMA by PE, despite cosmetic changes to laboratory indicators values. The mechanism of action of PE remains unclear, but may be through the clearance of CFH antibodies and inflammatory factors and the ability of fresh frozen plasma to provide soluble complement-regulatory proteins [8,11]. In addition, there is a lack of consensus on diagnostic criteria for TA-TMA, which makes it difficult to quickly and accurately identify the early stages of TA-TMA. The current mainstream diagnostic criteria include the BMT-CTN criteria, O criteria, Probable criteria, IWG criteria, and the criteria proposed by Jodele et al [12]. We performed a retrospective cohort study to analyze the efficacy of PE on TA-TMA and the factors affecting the efficacy, the prognostic survival of TA-TMA patients, and the factors affecting it. We also assessed the overall survival of different diagnostic criteria to help clinicians choose the diagnostic criteria for TA-TMA.
Material and Methods
ETHICS APPROVAL AND PATIENTS:
We included 589 patients with allo-HSCT from January 2018 to February 2022 at the First Medical Center of the PLA General Hospital. A total of 32 patients diagnosed with TA-TMA by different diagnostic criteria were included. This study was approved by the Ethics Committee of the Chinese PLA General Hospital and was conducted according to the Declaration of Helsinki.
DIAGNOSTIC CRITERIA FOR TA-TMA:
The laboratory results at the time of the TA-TMA diagnosis and upcoming treatment were compared to the following diagnostic criteria by reviewing the medical record system. Two individuals initially diagnosed with Probable TA-TMA had renal or neurological dysfunction at the time of relapse and met the CTN criteria and were excluded from the analysis of the diagnostic criteria.
O-TMA requires that each of the following be met: (1) lactate dehydrogenase (LDH) above the upper limit of normal; (2) the presence of ≥2 schistocytes per high-power field (HPF) in the blood smear; (3) platelet count <50×109/L or ≥50% decline in platelet count; (4) de novo anemia with a hemoglobin level below the lower limit of normal or anemia with increased transfusion requirements; (5) decreased serum haptoglobin; (6) negative Coomb’s test; and (7) the absence of coagulopathy [13].
CTN-TMA (definite-TMA) requires that each of the following be met: (1) the presence of ≥2 schistocytes per HPF in the blood smear (2) concurrent increased serum LDH above institutional baseline; (3) concurrent renal and/or neurologic dysfunction without other explanations; (4) negative direct and indirect Coombs test results [9].
Probable TA-TMA was defined as the presence of 3 of the 4 criteria except for renal and/or neurologic dysfunction [13].
Jodele TA-TMA is diagnosed by meeting >5 of 7 diagnostic markers: (1) LDH above the upper limit of normal; (2) schistocytes on blood smear; (3) platelet count <50×109/L or ≥50% decline in platelet count; (4) de novo anemia or increased transfusion requirements; (5) hypertension >99% for age (<18 years) or >140/90 (≥18 years of age); (6) proteinuria ≥30 mg/dL or random urine protein/creatinine ratio (rUPCR) ≥2 mg/mg; (7) elevated soluble terminal complement complex activity (plasma sC5b-9 above normal laboratory value) [14].
DATA COLLECTION AND DEFINITION:
Pre-transplant baseline variables tested included age, sex, disease type, donor type and HLA match, sex match, ABO match, conditioning regimen, time of onset, GVHD prior to TA-TMA diagnosis, comorbidities at diagnosis (including hypoproteinemia, bacterial infection, EBV/CMV infection, gastrointestinal bleeding, renal or neurological dysfunction), and laboratory indicators at diagnosis (including hemoglobin, platelets, LDH, schistocytes, blood pressure, creatinine, urine protein, total bilirubin). Early TA-TMA was defined as the appearance of TA-TMA within 100 days after HSCT, and late TA-TM was characterized by the appearance of TA-TMA more than 100 days after HSCT. Proteinuria was defined as random urine protein or urine protein-to-creatinine ratio greater than or equal to 2 mg/mg. Renal dysfunction is defined as either a doubling of serum creatinine from baseline, where the baseline is the creatinine taken before hydration and just before initiation of the conditioning regimen, or a 50% decrease in creatinine clearance from baseline.
TREATMENT MEASURES FOR TA-TMA:
In addition to the use of PE, other treatments include supportive therapies such as reduction/discontinuation of CNI/mTOR inhibitors, control of hypertension, transfusion of fresh frozen plasma, and treatment of comorbidities such as GVHD and infection that can promote the development of TA-TMA. All patients included in this study were subsequently reduced/discontinued from CNI/mTOR inhibitors at the time of TA-TMA diagnosis. The interval between stopping CNI or mTOR inhibitors and starting PE was 1 (0–46) day. Major drugs include CD20 monoclonal antibody, CD25 monoclonal antibody, mycophenolate mofetil (MMF), and glucocorticoids.
EFFICACY ASSESSMENT:
To define the efficacy of PE and its influencing factors, we compared those in remission and those not in remission after TA-TMA treatment with a view to concluding what kind of TA-TMA patients respond better to PE. Remission is defined as: (1) complete response (CR): satisfying both disappearance of schistocytes, any changes in mental status resolved, and LDH was normalized without red blood cell and platelet transfusion support; (2) non-response (NR): no improvement in laboratory indicators, still dependent on red blood cell and/or platelet transfusion support, or death from TA-TMA; (3) partial response (PR): Patients who did not meet the standards for either CR or NR were considered to have a partial response [15,16].
SURVIVAL ANALYSIS:
To identify predictors that had an impact on survival, we analyzed the characteristics of the survival and death groups according to survival or death at 6 months after TA-TMA diagnosis.
STATISTICAL ANALYSES:
Continuous data were described by median, range (minimum-maximum), or mean. Categorical data were described by frequency (percentage). The baseline characteristics and variables between 2 groups of patients were compared by
Results
CLINICAL CHARACTERISTICS OF PATIENTS:
The source of hematopoietic stem cells in our center was all peripheral blood hematopoietic stem cells, and 21 patients were treated with PE (Flowchart in Figure 1).
TA-TMA total population age of onset was 33.5 (13–61), of which a total of 22 (68.8%) were male. A total of 23 (71.9%) had early TA-TMA and 9 (28.1%) had late TA-TMA. Myeloid leukemia accounted for more primary features (56.3%). The majority of HSCT patients in our center used to receive busulfan (BU)-cyclophosphamide (CY) in combination with anti-thymocyte globulin (ATG) regimen for myeloablative conditioning. The other 3 patients received a reduced-intensity conditioning (RIC). The FluCY-ATG regimen was given to 1 patient with severe aplastic anemia (SAA) and the TBI/CY/ATG regimen was received by the remaining 2 patients. Of the 29 (90.6%) TA-TMA patients who received a conditioning regimen based on BU/CY + ATG, 6 were combined with ruxolitinib and decitabine, 1 with chidamide and ruxolitinib, 2 with chidamide, 2 with decitabine, and 1 with ruxolitinib. Almost all transplant recipients received CNI-based GVHD prophylaxis, while sirolimus was used less frequently in our center. For other features, haploidentical allo-HSCT was widely used in our center. Only 4 (12.5%) of the total TA-TMA population were unrelated donors and 2 (6.3%) were sibling allogeneic; 19 (59.4%) were sex-matched, 9 (28.1%) were ABO-matched, and 6 (18.8%) were HLA-matched.
TREATMENT RESPONSE OF PE:
Comparing baseline data (eg, sex, age, underlying disease, concomitant symptoms) between the PE and non-PE treatment groups, there were no significant differences in the characteristics between the 2 groups (Table 1), except for differences in time of onset (P=0.005) and Hb count at diagnosis (P=0.013). The PE group included 12 cases of NR (57.2), 4 cases of PR (19.0%), and 5 cases of CR (23.8%), while the non-PE group had 2 cases of NR (18.2%), 8 cases of PR (72.7%), and 1 case of CR (9.1%), with statistical differences in efficacy between the 2 groups (P=0.016). The non-PE group achieved a higher percentage of PR, while the PE group was able to achieve more CR.
In the univariate analysis affecting the efficacy of PE (Table 2), the time to onset of TA-TMA (67.0 vs 139.5, P=0.028), combined with elevated total bilirubin (11.1% vs 66.7%, P=0.024), combined renal or neurological dysfunction (11.1% vs 66.7%, P=0.024) differed between the remission and non-remission groups, while the number of plasma exchanges (6.0 vs 3.5, P=0.054) did not differ significantly between the remission and non-remission groups. However, in a multivariable analysis of PE efficacy, we found that the only factor affecting the response to PE treatment was the number of PE sessions (HR, 1.16; 95% CI, 1.00–1.36; P=0.047), although it may not be clinically significant.
OVERALL SURVIVAL:
The OS of patients with TA-TMA in this study was 50.0% (16/32) at 6 months and 59.4% (19/32) at 100 days after diagnosis (Kaplan-Meier curves are shown in Figure 2A), with a mean follow-up of 284 days (range, 67–1240). Four of them died of primary disease, 2 died of infectious shock, 6 died of multiorgan failure and TA-TMA (2 of these patients had a common cause of death including aGVHD and infection), and 4 were not recorded. There was no statistically significant difference between the PE and non-PE groups at 100-day OS (47.6% vs 81.8%, P=0.128) and 6-month OS (38.1% vs 72.7%, P=0.135).
The general characteristics of the surviving and deceased groups did not differ, and univariate analysis of other variables (Table 3) revealed that the deceased groups had the following characteristics compared to the surviving groups: (1) later onset of TA-TMA (139.5 [range, 29–591] vs 47 [range, 22–95], P=0.002); (2) combined III–IV aGVHD was more predominant (68.8% vs 12.5%, P=0.003); (3) total bilirubin was higher (36.4 [range, 6.5–329.0] vs 10.9 [range, 4.7–65.6], P=0.01); (4) platelet count was lower (13.5 [range, 3–72] vs 30.5 [range, 7–165], P=0.004); (5) a higher proportion of patients with hemoglobin below 70 g/L at diagnosis (81.2% vs 31.2%, P=0.023); (6) a higher proportion of patients with comorbid hypoproteinemia at diagnosis (87.5% vs 43.8%, P=0.011); (7) a higher proportion of patients with comorbid renal or neurological dysfunction (62.5% vs 12.5%, P=0.009). Kaplan-Meier curves suggested that late TA-TMA (0% vs 69.6%, P<0.001, Figure 2B), hypoproteinemia (33.3% vs 81.8%, P=0.004, Figure 2C), renal or neurological dysfunction (16.7% vs 70.0%, P<0.001, Figure 2D), III–IV aGVHD prior to TA-TMA diagnosis (15.4% vs 73.7%, P<0.001, Figure 3A), no response (14.3% vs 77.8%, P<0.001, Figure 3B, severe anemia (27.8% vs 78.6%, P=0.004, Figure 3C), and elevated TBIL (16.7% vs 70.0%, P<0.001, Figure 3D) were associated with poor survival at 6 months after TA-TMA.
We further performed a multivariable analysis (Table 4) of the survival prognosis of patients with TA-TMA and found that III–IV aGVHD prior to TA-TMA diagnosis (HR, 17.03; 95% CI, 2.76–105.3; P=0.002), renal or neurological dysfunction (HR, 7.4; 95% CI, 1.30–40.80; P=0.021), and the time to onset of TA-TMA (HR,1.02; 95% CI, 1.00–1.03; P=0.002) were independent risk factors for overall survival.
DISTRIBUTION OF PATIENTS WITH DIFFERENT TA-TMA DIAGNOSTIC CRITERIA:
The distribution of each definition of TMA is shown in Figure 4A, in which 22 patients met the O-TMA criteria, 24 patients met the Jodele criteria, 8 patients met the CTN criteria, and 14 patients met the Probable criteria.
EFFICACY AND OUTCOME OF PATIENT GROUPS WITH DIFFERENT DIAGNOSTIC CRITERIA:
Only 50% (11/22) of O-TMA patients achieved remission, of which only 1 achieved CR, while only 22.2% (2/9) of CTN-TMA patients achieved remission, 1 each with CR and PR. 71.4% (10/14) of Probable TA-TMA patients achieved remission, of which 1 achieved CR. Among Jodele TMA patients, 20.8% (5/24) achieved CR and the overall remission rate was 58.3% (14/24). The relatively good treatment remission rate in O-TMA patients can be explained by the better remission rate of Probable TA-TMA.
Probable TA-TMA had the highest survival rate with 78.6% (11/14) OS at 100 days and 64.3 (9/14) OS at 6 months. CTN-TMA had the lowest survival rate with 22.2% (2/9) OS at 100 days and 11.1% (1/9) OS at 6 months. In comparison, O-TMA had a 100-day OS of 54.5% (12/22) and a 6-month OS of 40.9% (9/22). The Jodele TMA 100-day OS was 66.7% (16/24) and the 6-month OS was 54.2% (13/24). The survival curves of TA-TMA by diagnostic criteria (including survival curves for both Jodele TMA and Probable TA-TMA patient groups) are illustrated in Figure 4B.
Discussion
This study is the first to specifically assess the efficacy and treatment outcome of plasma exchange for TA-TMA in the context of each current diagnostic criteria at the same time. Independent risk factors for overall survival in TA-TMA were identified: the combination of renal or neurological dysfunction, III–IV aGVHD prior to TA-TMA, and the time to onset of TA-TMA. In addition, time to onset of TA-TMA (67.0 vs 139.5, P=0.028), combined with elevated total bilirubin (11.1% vs 66.7%, P=0.024), and combined renal or neurological dysfunction (11.1% vs 66.7%, P=0.024) were factors that can affect the response to PE treatment. The multivariate analysis of PE efficacy showed that the only significant variable was the number of sessions of PE. This may be biased, since those who deteriorated early would have received fewer sessions.
According to research by Sudhanshu Mulay et al, patients with early TA-TMA had a worse prognosis and treatment outcome than those with late TA-TMA [17]. In contrast, our investigation revealed a steadily rising risk of death as the duration of TA-TMA onset increased (remission rates and 30-day OS in the study by Mulay et al were not statistically significant). It is possible that the study was based on a population of TA-TMA patients with different diagnostic criteria, resulting in poor survival in patients with delayed diagnosis. Moreover, aGVHD as a risk factor for TA-TMA in the study is consistent with a large body of previous literature [18,19].
Existing guidelines recommend the withdrawal of CNIs as the first step in controlling TA-TMA [20]. The literature reports that 27% of patients who discontinued immunosuppression alone achieved hematologic remission within 90 days of diagnosis and 57% survived. A prospective study evaluated the benefit of PE in patients with TA-TMA; the treatment response rate for TA-TMA patients who initiated PE therapy immediately after discontinuing cyclosporine was 64%. It was also concluded that early initiation of treatment may be associated with better outcomes [21]. In contrast, 38.1% of the patients in the PE treatment group in our study experienced remission within 90 days of diagnosis, and the median length of remission for the 9 TA-TMA patients who experienced remission following PE treatment was 5 days (range, 2–43). This suggests that PE can indeed relieve clinical symptoms in the short term. The Blood and Marrow Transplant Clinical Trials Network Toxicity Committee summarized studies from 1991 to 2003 and reported that the efficacy of PE for TA-TMA was 36.5% (range, 0–80%) and the mortality rate was as high as 80% (range, 44–100%) [9]. Laskin et al analyzed clinical studies of PE for TA-TMA from 2003 to 2010 and showed a median efficacy of 59% (range, 27–80%) and a mortality rate of 56% (range, 20–92%) [8]. A multitude of other reports in the last decade have also reported PE has no overall impact on survival [22,23]. In our study, the remission rate of TA-TMA patients treated with PE was 42.8%, and the overall survival at 100 days and 6 months after TA-TMA was 47.6% and 38.1%, respectively. There was no significant difference in overall survival between the PE and non-PE groups. As several other studies have similarly shown, the application of PE early in the TA-TMA process can terminate the pathological process leading to endothelial injury and thus can improve laboratory indicators (eg, LDH, haptoglobin, platelets). However, it does not modify the subsequent persistent tissue damage and enhance OS [8,22], and the complications of PE should not be ignored [24].
Because of the role of complement activation in the pathogenesis of TA-TMA, the complement C5 inhibitor eculizumab has shown good efficacy in TA-TMA. In a prospective study with eculizumab in pediatric patients with high-risk TA-TMA published by Jodele et al, 64% of patients achieved remission, and 66% survived at 1 year after HSCT [16]. According to the published research, the OS of TA-TMA patients treated with eculizumab is 33–60%, with a median remission rate of 67% (range 50–93%) [14,25–30]. Based on these studies, eculizumab is considered a first-line treatment for TA-TMA patients with evidence of complement activation. However, a recent article compared eculizumab with conventional therapy in a total of 39 adults with TA-TMA (including defibrotide alone or in combination with PE or rituximab). Although the eculizumab-treated group experienced an excellent response and much greater remission rates than the conventional treatment group, the overall survival rate at the end of a median follow-up of 30 weeks was only 33% [12]. In addition, given that most complement therapy studies are retrospective studies focusing on pediatric populations, further studies are needed to validate the improvement of complement blockers on prognostic survival in TA-TMA. Moreover, because complement blockers are costly, PE can still be a therapeutic strategy for TA-TMA.
A new TA-TMA diagnostic and treatment algorithm proposed in a recent review suggests that daily PE treatment or combined immunosuppression should be administered early in patients diagnosed with high-risk TA-TMA and the presence of antibodies to CFH or ADAMTS13 [12]. This demonstrates the therapeutic benefit of PE for high-risk TA-TMA and implies the clinical utility of antibodies to CFH or ADAMTS13 testing.
Since the IWG-TMA demands schistocytes 8/HPF, our study did not examine the IWG criteria (only 2 patients met the criteria). As previous studies have shown that although schistocytes are often used as a feature of microangiopathy, they are not a predictor of early TMA diagnosis. Instead, many clinically significant TMA may go unnoticed [31]. In our study, Probable TA-TMA had the highest survival rate, while CTN-TMA had the lowest. We also found that the time to onset of Probable criteria TMA was the shortest of the 4 diagnostic criteria. Combined with our previous findings on the time to onset of TMA diagnosis as a risk factor for overall survival, Probable TA-TMA significantly improved prognostic survival through early detection of patients with TA-TMA. This is consistent with the extensive previous literature reporting that early treatment significantly improves the prognosis of TA-TMA patients. Acute renal or neurological dysfunction is less common at the onset of TA-TMA [32], and once present, the patient is already in critical condition. The CTN-TMA requires the presence of renal and/or neurological dysfunction, which limits the sensitivity of the diagnosis and leads to poor survival due to failure to intervene early. In comparison, the newly proposed Jodele criteria include the first introduction of complement activation marker serum soluble C5b-9 (sC5b-9) levels, histological evidence, hypertension, and proteinuria, which were more widely recognized [14,33]. The sC5b9 level was not measured in our center. Therefore, our study of Jodele TMA patients had a delayed diagnosis. In addition, we unexpectedly found that the population of patients who met both the Jodele criteria and Probable TA-TMA had significantly higher survival than the other criteria. This suggests that the combination of Jodele and Probable TA-TMA criteria may contribute to earlier diagnosis and improved prognosis.
Previous studies recommended PE with 1 to 1.5 times the plasma volume (40–60 ml/kg body weight) daily for 1 to 2 weeks, slowly reducing the dose to 1 every other day for 1–2 weeks, then 2–3 times a week for 1–2 weeks. If laboratory findings indicate recurrence of microangiopathy, daily PE should be resumed [33]. Most of the patients in our center underwent daily PE within the first week. However, some of them failed to receive regular treatment, which resulted in decreased efficacy and poorer survival. Bleeding tendency, allergic reactions, hypotension, and cardiac insufficiency are a few reasons why routine plasma exchange is not performed [34,35]. Another possible explanation for the failure of TMA treatment is that most of the patients we included had some delay in diagnosis, as evidenced by high median LDH levels and low platelet and hemoglobin levels at the onset of TMA.
We compared the PE group with the non-PE group by TMA index [32] and BATAP prognostic stratification [36]. The distribution of the PE group was mainly intermediate- and high-risk, while the non-PE group was mainly low- and intermediate-risk (Table 5). A major limitation in this study is its retrospective nature. There may be some bias because clinicians frequently encourage high-risk patients to actively pursue PE therapy, which led to a decrease in the number of high-risk TA-TMA patients in the non-PE group. As a temporary therapy option for TA-TMA when complement blockers are unavailable, the value of PE is confined to short-term improvement of laboratory values and symptom relief. There is no appreciable predictive survival improvement. After testing wherever possible for antibodies to CFH or ADAMTS13, physicians should consider whether to use PE and be alert to any complications related to PE.
Conclusions
We found that PE can temporarily alleviate TA-TMA patients’ clinical symptoms and improve laboratory indicators. It does not, however, significantly increase overall survival. The only factor affecting the response to PE treatment was the number of PE sessions. Furthermore, the combination of renal or neurological dysfunction, III–IV aGVHD prior to TA-TMA, and the time to onset of TA-TMA are independent predictors of survival in TA-TMA patients. We found that Probable TA-TMA had the highest survival rate, but the Jodele criteria are expected to diagnose earlier and provide the greatest benefit to patients. There is a need for further large prospective trials in adults to identify the population more suitable for PE treatment of TA-TMA.
Figures
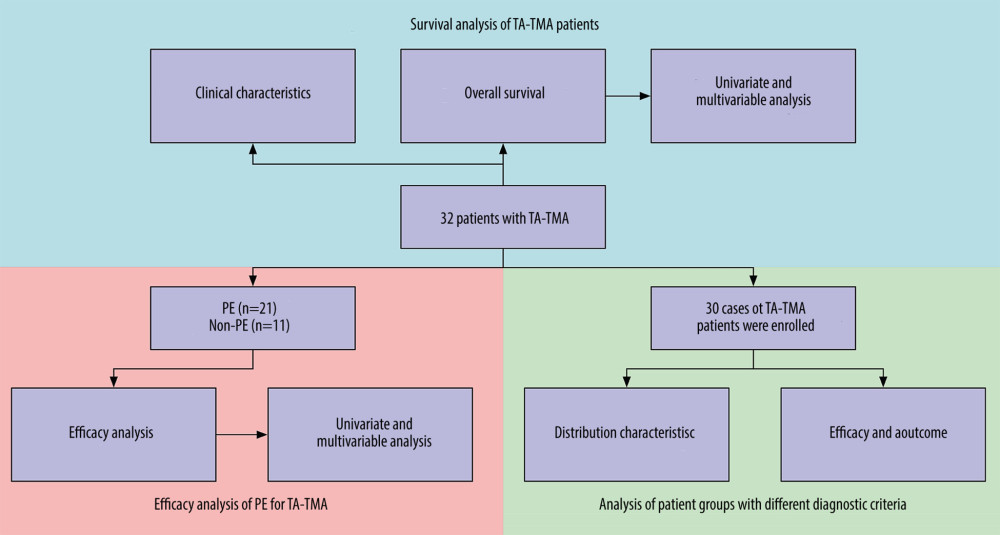 Figure 1. Flow chart of the study.
Figure 1. Flow chart of the study. 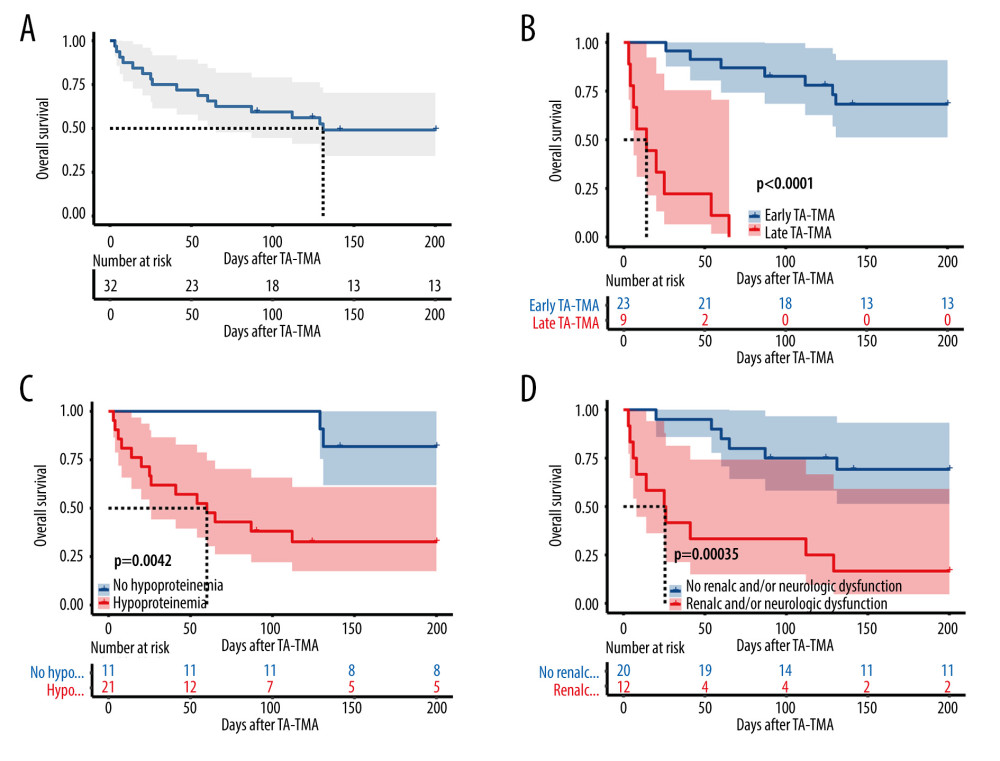 Figure 2. Overall survival rate of TA-TMA patients. Kaplan-Meier curves were used to calculate the survival rate at 6 months after TA-TMA using the log-rank test. (A) Total subjects. (B) Early TA-TMA and late TA-TMA (C) Hypoproteinemia. (D) Renal or neurological dysfunction. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria).
Figure 2. Overall survival rate of TA-TMA patients. Kaplan-Meier curves were used to calculate the survival rate at 6 months after TA-TMA using the log-rank test. (A) Total subjects. (B) Early TA-TMA and late TA-TMA (C) Hypoproteinemia. (D) Renal or neurological dysfunction. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria). 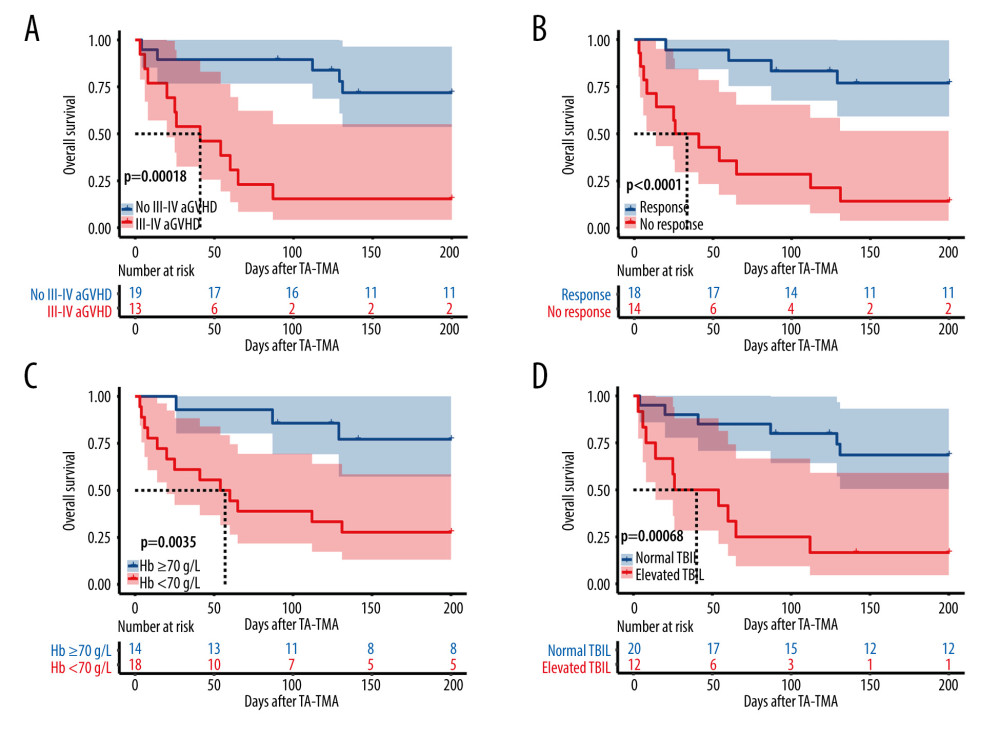 Figure 3. (A) III–IV aGVHD prior to TA-TMA diagnosis. (B) Response. (C) Hemoglobin. (D) Total bilirubin. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria).
Figure 3. (A) III–IV aGVHD prior to TA-TMA diagnosis. (B) Response. (C) Hemoglobin. (D) Total bilirubin. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria). 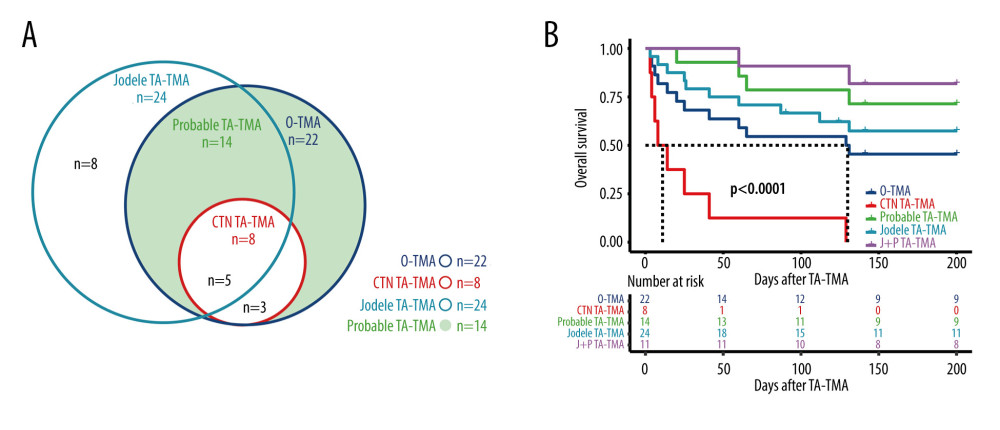 Figure 4. (A) Distribution of TA-TMA patients for various diagnostic criteria (B) Kaplan-Meier curves for patient groups with different diagnostic criteria. J+P TMA: the patient groups who met both Jodele criteria and Probable TMA. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria).
Figure 4. (A) Distribution of TA-TMA patients for various diagnostic criteria (B) Kaplan-Meier curves for patient groups with different diagnostic criteria. J+P TMA: the patient groups who met both Jodele criteria and Probable TMA. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria). Tables
Table 1. Clinical characteristics of the TA-TMA.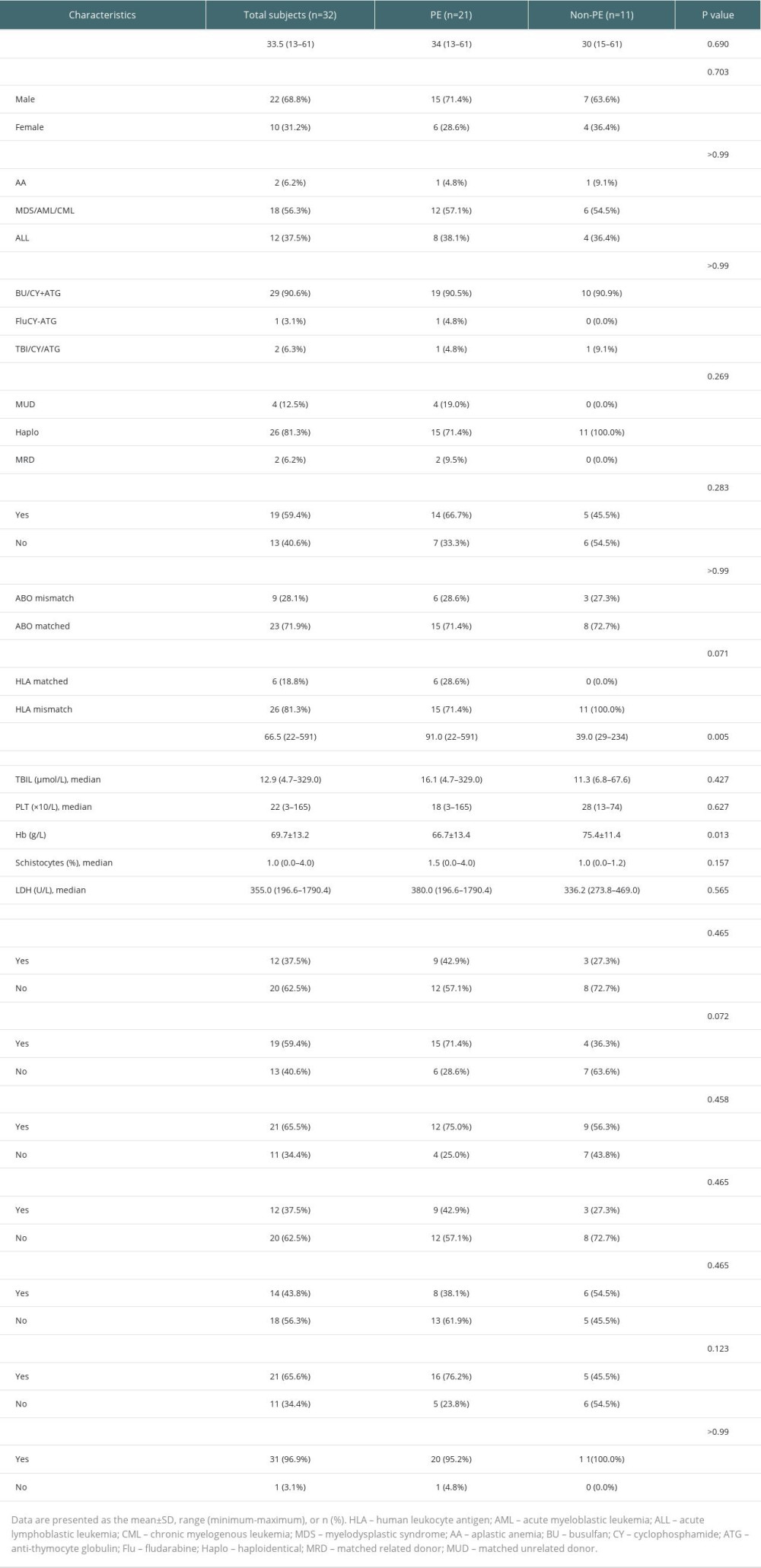 Table 2. Univariate analysis of the efficacy of PE for TA-TMA.
Table 2. Univariate analysis of the efficacy of PE for TA-TMA.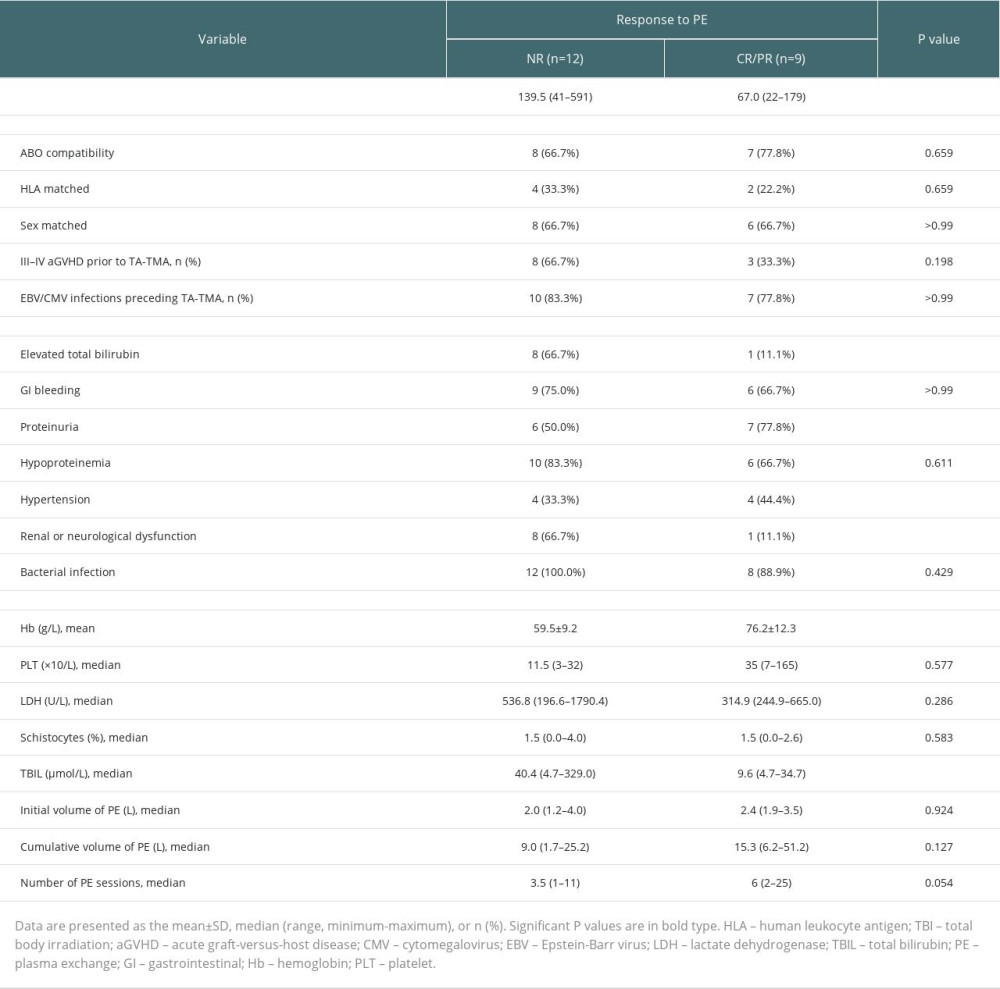 Table 3. Survival group vs deceased group.
Table 3. Survival group vs deceased group.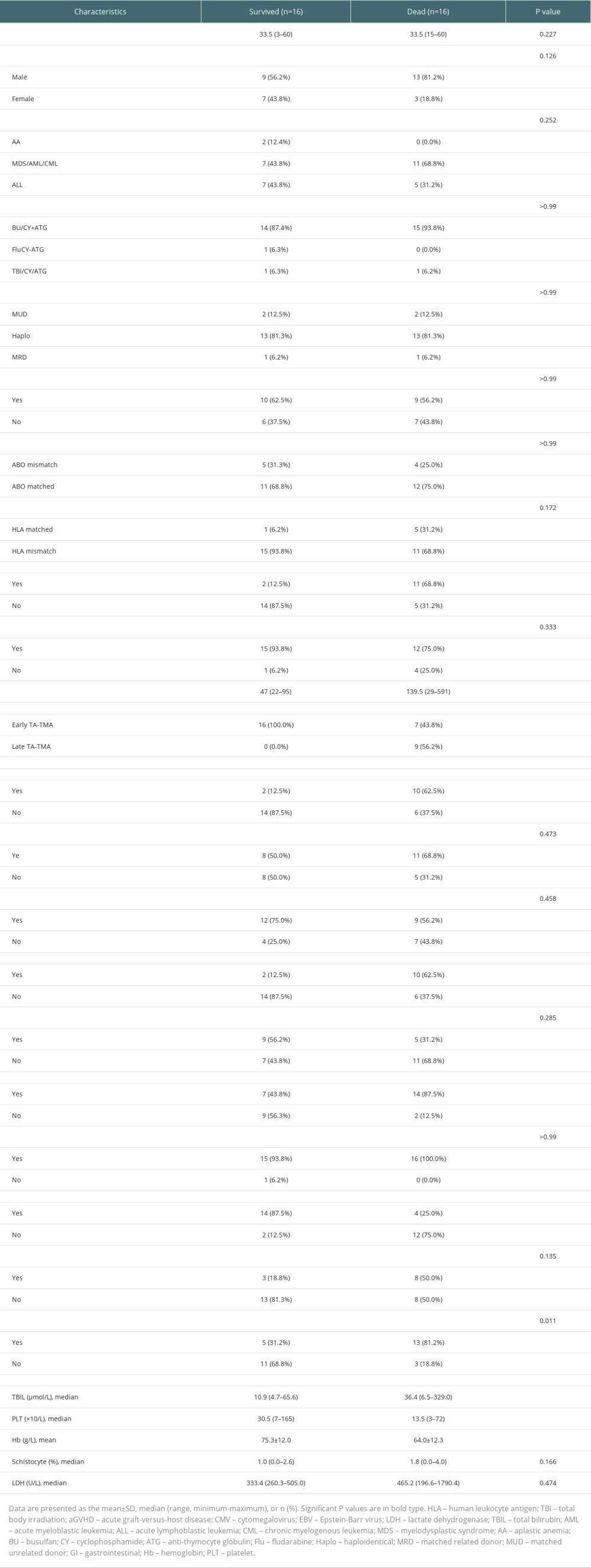 Table 4. Multivariable analyses affecting the overall survival of TA-TMA patients.
Table 4. Multivariable analyses affecting the overall survival of TA-TMA patients.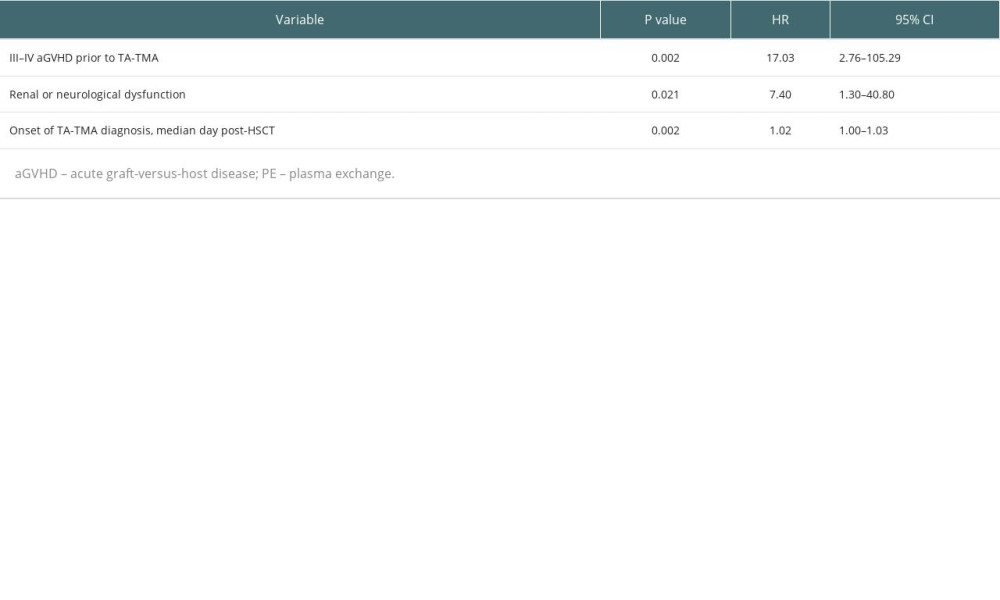 Table 5. Risk stratification distribution of PE and non-PE groups.
Table 5. Risk stratification distribution of PE and non-PE groups.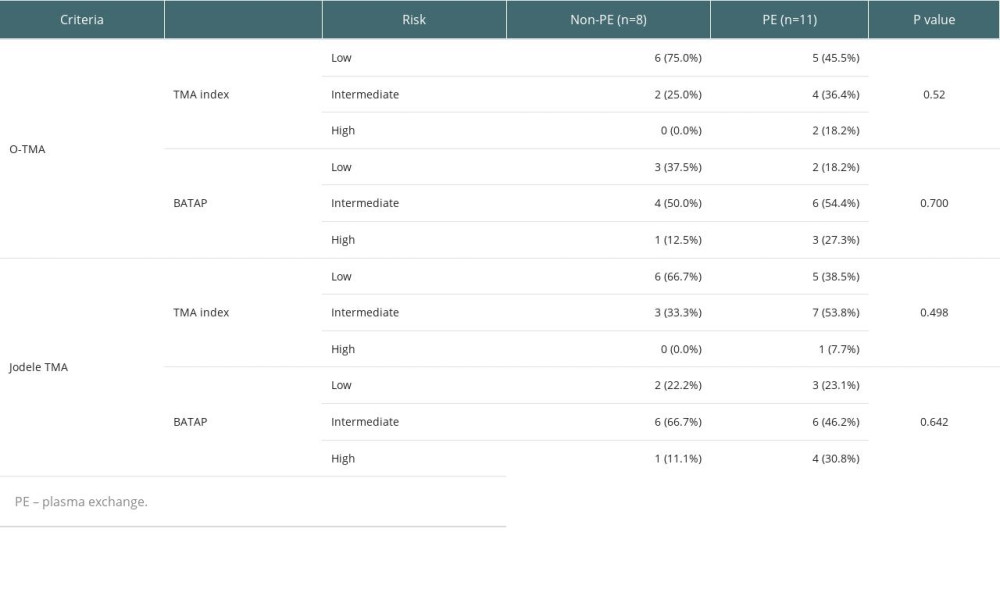
References
1. Wanchoo R, Bayer RL, Bassil C, Emerging concepts in hematopoietic stem cell transplantation-associated renal thrombotic microangiopathy and prospects for new treatments: Am J Kidney Dis, 2018; 72(6); 857-65
2. Warren M, Jodele S, Dandoy C, A Complete histologic approach to gastrointestinal biopsy from hematopoietic stem cell transplant patients with evidence of transplant-associated gastrointestinal thrombotic microangiopathy: Arch Pathol Lab Med, 2017; 141(11); 1558-66
3. Yamada R, Nemoto T, Ohashi K, Distribution of transplantation-associated thrombotic microangiopathy (TA-TMA) and comparison between renal TA-TMA and intestinal TA-TMA: Autopsy study: Biol Blood Marrow Transplant, 2020; 26(1); 178-88
4. Meri S, Bunjes D, Cofiell R, The role of complement in HSCT-TMA: Basic science to clinical practice: Adv Ther, 2022; 39(9); 3896-915
5. Jodele S, Sabulski A, Transplant-associated thrombotic microangiopathy: Elucidating prevention strategies and identifying high-risk patients: Expert Rev Hematol, 2021; 14(8); 751-63
6. Carmona A, Díaz-Ricart M, Palomo M, Distinct deleterious effects of cyclosporine and tacrolimus and combined tacrolimus-sirolimus on endothelial cells: Protective effect of defibrotide: Biol Blood Marrow Transplant, 2013; 19(10); 1439-45
7. Eissner G, Multhoff G, Gerbitz A, Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: Protective effect of defibrotide: Blood, 2002; 100(1); 334-40
8. Laskin BL, Goebel J, Davies SM, Small vessels, big trouble in the kidneys and beyond: Hematopoietic stem cell transplantation-associated thrombotic microangiopathy: Blood, 2011; 118(6); 1452-62
9. Ho VT, Cutler C, Carter S, Blood and marrow transplant clinical trials network toxicity committee consensus summary: Thrombotic microangiopathy after hematopoietic stem cell transplantation: Biol Blood Marrow Transplant, 2005; 11(8); 571-75
10. Oran B, Donato M, Aleman A, Transplant-associated microangiopathy in patients receiving tacrolimus following allogeneic stem cell transplantation: Risk factors and response to treatment: Biol Blood Marrow Transplant, 2007; 13(4); 469-77
11. Chapin J, Shore T, Forsberg P, Hematopoietic transplant-associated thrombotic microangiopathy: Case report and review of diagnosis and treatments [J]: Clin Adv Hematol Oncol, 2014; 12(9); 565-73
12. Young JA, Pallas CR, Knovich MA, Transplant-associated thrombotic microangiopathy: Theoretical considerations and a practical approach to an unrefined diagnosis: Bone Marrow Transplant, 2021; 56(8); 1805-17
13. Cho BS, Yahng SA, Lee SE, Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation: Transplantation, 2010; 90(8); 918-26
14. Jodele S, Dandoy CE, Myers KC, New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy: Transfus Apher Sci, 2016; 54(2); 181-90
15. Kennedy GA, Kearey N, Bleakley S, Transplantation-associated thrombotic microangiopathy: Effect of concomitant GVHD on efficacy of therapeutic plasma exchange: Bone Marrow Transplant, 2010; 45(4); 699-704
16. Jodele S, Dandoy CE, Lane A, Complement blockade for TA-TMA: Lessons learned from a large pediatric cohort treated with eculizumab: Blood, 2020; 135(13); 1049-57
17. Mulay S, Kreuter JD, Bryant SC, Outcomes of plasma exchange in patients with transplant-associated thrombotic microangiopathy based on time of presentation since transplant: J Clin Apher, 2015; 30(3); 147-53
18. Kraft S, Bollinger N, Bodenmann B, High mortality in hematopoietic stem cell transplant-associated thrombotic microangiopathy with and without concomitant acute graft-versus-host disease: Bone Marrow Transplant, 2019; 54(4); 540-48
19. Zeisbrich M, Becker N, Benner A, Transplant-associated thrombotic microangiopathy is an endothelial complication associated with refractoriness of acute GvHD: Bone Marrow Transplant, 2017; 52(10); 1399-405
20. Matsui H, Arai Y, Imoto H, Risk factors and appropriate therapeutic strategies for thrombotic microangiopathy after allogeneic HSCT: Blood Adv, 2020; 4(13); 3169-79
21. Worel N, Greinix HT, Leitner G, ABO-incompatible allogeneic hematopoietic stem cell transplantation following reduced-intensity conditioning: Close association with transplant-associated microangiopathy: Transfus Apher Sci, 2007; 36(3); 297-304
22. Jodele S, Laskin BL, Goebel J, Does early initiation of therapeutic plasma exchange improve outcome in pediatric stem cell transplant-associated thrombotic microangiopathy?: Transfusion, 2013; 53(3); 661-67
23. Riedl M, Fakhouri F, Le Quintrec M, Spectrum of complement-mediated thrombotic microangiopathies: Pathogenetic insights identifying novel treatment approaches: Semin Thromb Hemost, 2014; 40(4); 444-64
24. Nguyen L, Terrell DR, Duvall D, Complications of plasma exchange in patients treated for thrombotic thrombocytopenic purpura. IV. An additional study of 43 consecutive patients, 2005 to 2008: Transfusion, 2009; 49(2); 392-94
25. De Fontbrune FS, Galambrun C, Sirvent A, Use of eculizumab in patients with allogeneic stem cell transplant-associated thrombotic microangiopathy: A study from the SFGM-TC: Transplantation, 2015; 99(9); 1953-59
26. Vasu S, Wu H, Satoskar A, Eculizumab therapy in adults with allogeneic hematopoietic cell transplant-associated thrombotic microangiopathy: Bone Marrow Transplant, 2016; 51(9); 1241-44
27. Rudoni J, Jan A, Hosing C, Eculizumab for transplant-associated thrombotic microangiopathy in adult allogeneic stem cell transplant recipients: Eur J Haematol, 2018; 101(3); 389-98
28. Bohl SR, Kuchenbauer F, Von Harsdorf S, Thrombotic microangiopathy after allogeneic stem cell transplantation: A comparison of eculizumab therapy and conventional therapy: Biol Blood Marrow Transplant, 2017; 23(12); 2172-77
29. Epperla N, Hemauer K, Hamadani M, Impact of treatment and outcomes for patients with posttransplant drug-associated thrombotic microangiopathy: Transfusion, 2017; 57(11); 2775-81
30. Jodele S, Fukuda T, Vinks A, Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy: Biol Blood Marrow Transplant, 2014; 20(4); 518-25
31. Murphree CR, Nguyen NN, Shatzel JJ, Biopsy-proven thrombotic microangiopathy without schistocytosis on peripheral blood smear: A cautionary tale: Am J Hematol, 2019; 94(9); E234-E37
32. Uderzo C, Bonanomi S, Busca A, Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation: Transplantation, 2006; 82(5); 638-44
33. Jodele S, Laskin BL, Dandoy CE, A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury: Blood Rev, 2015; 29(3); 191-204
34. Howard MA, Williams LA, Terrell DR, Complications of plasma exchange in patients treated for clinically suspected thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: Transfusion, 2006; 46(1); 154-56
35. Batts ED, Lazarus HM, Diagnosis and treatment of transplantation-associated thrombotic microangiopathy: Real progress or are we still waiting?: Bone Marrow Transplant, 2007; 40(8); 709-19
36. Zhao P, Wu YJ, He Y, A prognostic model (BATAP) with external validation for patients with transplant-associated thrombotic microangiopathy: Blood Adv, 2021; 5(24); 5479-89
Figures
 Figure 1. Flow chart of the study.
Figure 1. Flow chart of the study. Figure 2. Overall survival rate of TA-TMA patients. Kaplan-Meier curves were used to calculate the survival rate at 6 months after TA-TMA using the log-rank test. (A) Total subjects. (B) Early TA-TMA and late TA-TMA (C) Hypoproteinemia. (D) Renal or neurological dysfunction. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria).
Figure 2. Overall survival rate of TA-TMA patients. Kaplan-Meier curves were used to calculate the survival rate at 6 months after TA-TMA using the log-rank test. (A) Total subjects. (B) Early TA-TMA and late TA-TMA (C) Hypoproteinemia. (D) Renal or neurological dysfunction. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria). Figure 3. (A) III–IV aGVHD prior to TA-TMA diagnosis. (B) Response. (C) Hemoglobin. (D) Total bilirubin. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria).
Figure 3. (A) III–IV aGVHD prior to TA-TMA diagnosis. (B) Response. (C) Hemoglobin. (D) Total bilirubin. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria). Figure 4. (A) Distribution of TA-TMA patients for various diagnostic criteria (B) Kaplan-Meier curves for patient groups with different diagnostic criteria. J+P TMA: the patient groups who met both Jodele criteria and Probable TMA. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria).
Figure 4. (A) Distribution of TA-TMA patients for various diagnostic criteria (B) Kaplan-Meier curves for patient groups with different diagnostic criteria. J+P TMA: the patient groups who met both Jodele criteria and Probable TMA. R Core Team (2021). (R Foundation for Statistical, Computing, Vienna, Austria). Tables
 Table 1. Clinical characteristics of the TA-TMA.
Table 1. Clinical characteristics of the TA-TMA. Table 2. Univariate analysis of the efficacy of PE for TA-TMA.
Table 2. Univariate analysis of the efficacy of PE for TA-TMA. Table 3. Survival group vs deceased group.
Table 3. Survival group vs deceased group. Table 4. Multivariable analyses affecting the overall survival of TA-TMA patients.
Table 4. Multivariable analyses affecting the overall survival of TA-TMA patients. Table 5. Risk stratification distribution of PE and non-PE groups.
Table 5. Risk stratification distribution of PE and non-PE groups. Table 1. Clinical characteristics of the TA-TMA.
Table 1. Clinical characteristics of the TA-TMA. Table 2. Univariate analysis of the efficacy of PE for TA-TMA.
Table 2. Univariate analysis of the efficacy of PE for TA-TMA. Table 3. Survival group vs deceased group.
Table 3. Survival group vs deceased group. Table 4. Multivariable analyses affecting the overall survival of TA-TMA patients.
Table 4. Multivariable analyses affecting the overall survival of TA-TMA patients. Table 5. Risk stratification distribution of PE and non-PE groups.
Table 5. Risk stratification distribution of PE and non-PE groups. In Press
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








