21 November 2023: Original Paper
Predictors of Long-Term Outcomes After Liver Transplantation for Unresectable Metastatic Neuroendocrine Tumors
Mikołaj KuncewiczDOI: 10.12659/AOT.941212
Ann Transplant 2023; 28:e941212
Abstract
BACKGROUND: Malignant and benign neuroendocrine tumors (NET) share many histopathological features. Liver transplantation (LT) is one of the liver-directed therapies for neuroendocrine liver metastases (NELM). The aim of this study was to determine the outcomes of patients undergoing LT for NELM.
MATERIAL AND METHODS: This was a retrospective study that included 19 patients who underwent LT for unresectable NELM between December 1989 and December 2022 in the Department of General, Transplant, and Liver Surgery of the Medical University of Warsaw. Kaplan-Meier estimator and Cox proportional hazards regression were used for statistical analyses.
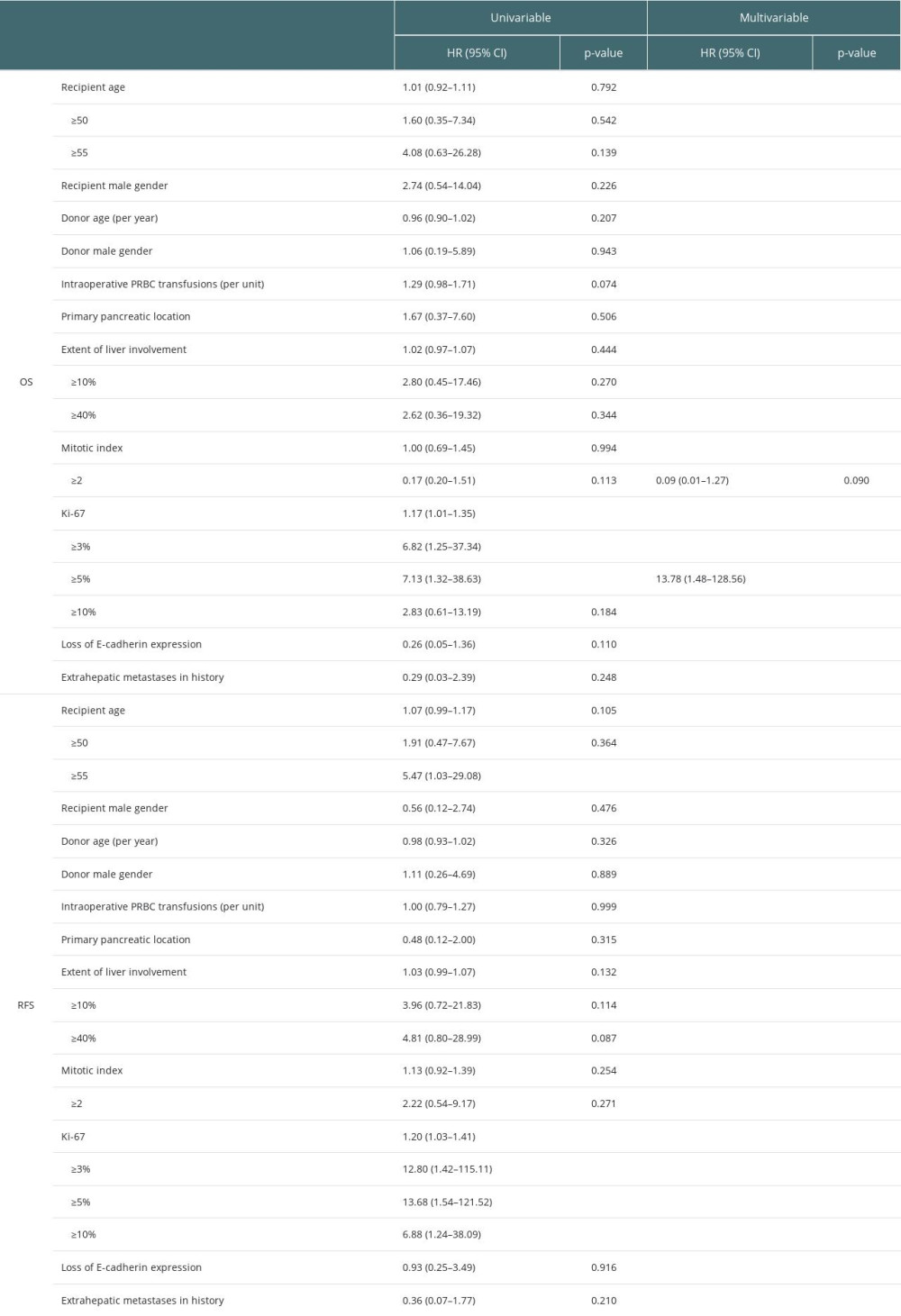
RESULTS: The primary tumor was located most frequently in the pancreas. The median follow-up was 72.5 months. The overall survival (OS) was 94.7%, 88.0%, 88.0%, 70.4%, and 49.3% after 1, 3, 5, 10, and 15 years, respectively. Accordingly, the recurrence-free survival (RFS) rates were 93.8%, 72.9%, 64.8%, 27.8%, and 27.8% after 1, 3, 5, 10, and 15 years, respectively. Ki-67 index ≥5% was found as a risk factor for both worse OS (hazard ratio (HR) 7.13, 95% confidence intervals (95% CI) 1.32-38.63, P=0.023) and RFS (HR 13.68, 95% CI 1.54-121.52, P=0.019). Recipient age ≥55 years was a risk factor for worse RFS (P=0.046, HR 5.47, 95% CI 1.03-29.08). Multivariable analysis revealed Ki-67 ≥5% as the sole independent factor for worse OS (HR 13.78, 95% CI 1.48-128.56, P=0.021).
CONCLUSIONS: Patients with unresectable NELM achieve great OS and satisfying RFS after LT. The risk factors associated with worse outcomes are attributed to primary tumor aggressiveness.
Keywords: Liver Transplantation, Neoplasm Metastasis, neuroendocrine tumors, surgical oncology, Liver Neoplasms
Background
Neuroendocrine tumors (NET) are a heterogeneous group of tumors with an incidence of more than 5 per 100 000 people [1]. The neoplasms derive from neuroendocrine cells present in the bronchopulmonary complex, gastrointestinal tract, and pancreas [2]. There is a huge variety of clinical presentations within the group. The histological appearance of NET is not strictly associated with metastatic potential. Disease progression is often asymptomatic for nonfunctioning tumors and manifest with metastases. Functioning NET produce hormones resulting in endocrinopathies, which worsen quality of life and may be life-threatening. The burden of both functioning and nonfunctioning NET relies on the combined size of tumors. Lesions can metastasize to different organs, including the liver, bones, and lungs. In the vast majority of cases, NET are detected in the metastatic phase (40–80%), of which neuroendocrine metastases to the liver (NELM) are the most common [3].
The goal of treatment is to prolong overall and symptom-free survival. Treatment of NELM relies on cytoreduction, achieved with liver-directed therapy and pharmacological treatment. Pharmacological treatments include somatostatin analogs (SSAs), everolimus, sunitinib, and peptide receptor radionuclide therapy (PRRT) [4]. Invasive methods such as transarterial chemoembolization (TACE), transarterial radioembolization (TARE), radiofrequency ablation (RFA), cryoablation, brachytherapy, and hepatic artery embolization are commonly applied [5–8]. However, the 5-year overall survival (OS) reported for these treatment modalities vary from 17% to 57% for selected patients [9–11]. In a study by Roche et al, the 5-year OS for patients with NELM after TACE was reported to be 83% [12]. The median RFS for NELM can also vary from 11 to 15 months [6,13]. Currently, liver resection (LR) allows the best treatment results in patients with resectable disease [14]. However, due to tumor biology and insufficient preoperative imaging techniques, complete cytoreduction is difficult to obtain, especially with bilobar liver metastases. A multicenter study by Eshmuminov et al found the 5-year OS of patients treated with LR was 68.8%, with a 5-year RFS of 18.1% [15]. Liver transplantation (LT) is generally regarded as the best method for complete tumor clearance. However, strict selection of patients is needed to outweigh risks by potential benefits [15]. The number of donors is insufficient; therefore, appropriate organ allocation is necessary [16,17]. The Milan Criteria, United Network for Organ Sharing guidelines, or European Neuroendocrine Tumor Society criteria can be used to assess patient eligibility for LT [18].
Direct comparison of the various treatment modalities is difficult. Patients undergoing LT for NELM usually have already benefited from other treatment modalities prior to transplantation. Nevertheless, factors for selection of an appropriate therapy are unclear [19]. That is especially meaningful in light of newly reported research stating that selected patients undergoing LT can achieve better outcomes than parallel patients undergoing LR [15].
Among risk factors, the Ki-67 index is reported to be the most important for patient stratification. However, an exact value has yet to be determined, as it varies among studies [15,20–22]. The aim of the present study was to assess long-term outcomes after LT in patients with NELM and to define prognostic factors.
Material and Methods
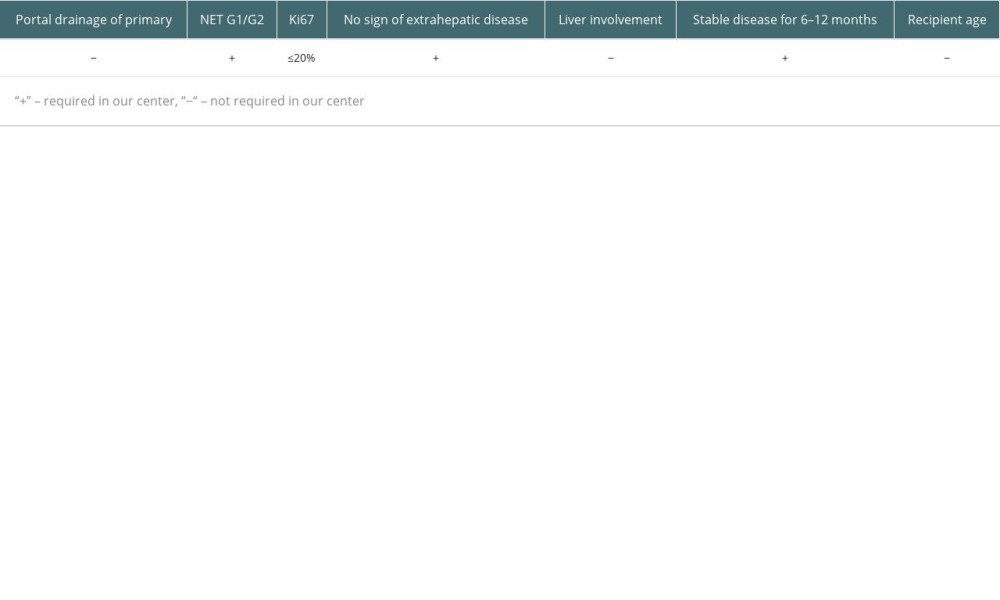
This was a retrospective observational study. There were 2680 LTs performed in the Department of General, Transplant, and Liver Surgery of the Medical University of Warsaw between December 1989 and December 2022. According to the internal register of the department, 19 (0.7%) of them were identified as primary transplantations for NELM. The eligibility criteria in our center require grade 1 (G1) or grade 2 (G2) in the 2019 World Health Organization (WHO) NEN Classification, stable disease for 6–12 months, and no sign of extrahepatic disease at the time of LT, but patients who had previously removed metastases are accepted. Contrary to the practice of some centers, we do not require portal drainage of the primary lesion, and older patient age is not a contraindication nor are liver involvement or Ki-67 value, as long as it does not exceed 20%, which is defined as G1 or G2. During patient selection for LT, results based on primary lesion or biopsy of liver metastases were used to assess the Ki-67 value. However, further evaluations of post-transplantation outcomes were based on pathological examination of the explanted liver as the most reliable and suitable for description of neoplasm aggressiveness. The criteria are summarized in Table 1 [15]. The lesions were defined as unresectable during multidisciplinary team (MDT) meetings by the experienced hepatobiliary surgeons and radiologists, based on computed tomography (CT), magnetic resonance imaging (MRI), or intraoperative finding of liver dissemination. During analysis, associations with outcomes were tested for numerous factors listed in Table 2. Liver involvement was defined as the volume revealed in imaging technique lesions relative to the total volume of the liver. Volumetry was based on either CT or MRI scans. E-cadherin expression was characterized by the previously described histoscore, determined by the pathologist by multiplying the intensity of staining (negative – 0; weak – 1; moderate – 2; strong – 3) and percentage of cells with positive staining (0–5% – 0; 6–25% – 1; 26–50% – 2; 51–75% – 3; 76–100% – 4) [20,23]. Scores of 8 or higher were accepted as intact E-cadherin expression, while lower values signified a loss of expression (Figure 1).
In most cases, the classic technique of cavo-caval anastomosis was applied, with end-to-end biliary anastomosis. Prior to LT, most patients had undergone numerous types of systemic treatment and liver resections, along with other modalities (Table 3). Data on disease recurrence were censored on the last follow-up visit or control imaging examination. OS was set as the primary outcome and RFS as the secondary outcome. Kaplan-Meier method was used for survival estimate calculations. The Cox proportional hazards ratio was applied for risk factor analysis. Univariable analyses were performed. Subsequently, a model for multivariable analysis was built with backward elimination out of factors, which obtained
Results
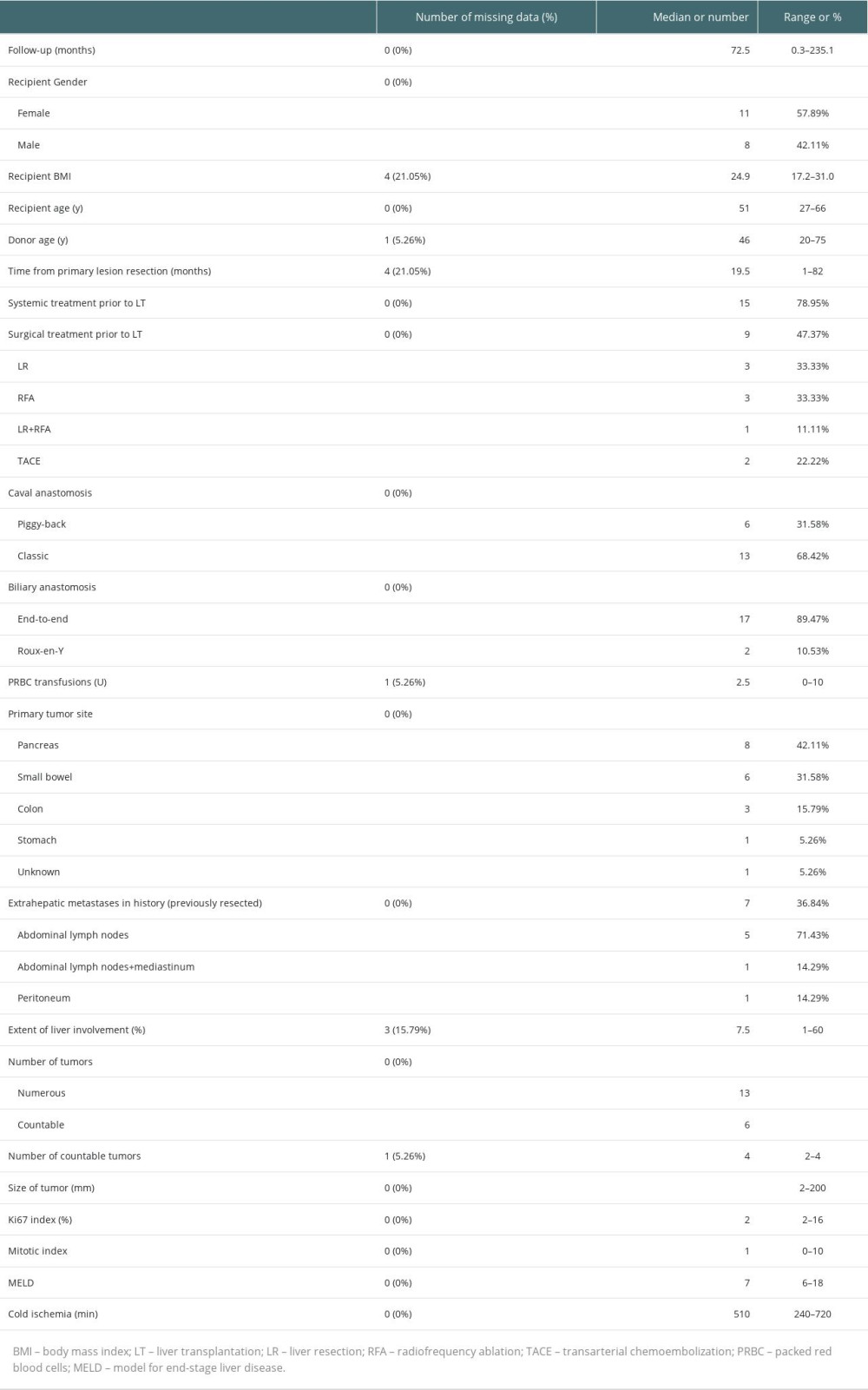
Characteristics of the 19 recipients are presented in Table 3. There were 11 (57.9%) females and 8 (42.1%) males. The primary tumor was located most frequently in the pancreas (42.1%), followed by the small bowel (31.6%). The median follow-up was 72.5 months (0.3–235.1 months). There were 3 (17.6%) patients beyond the Milan Criteria. During the observation period, there were 3 (15.8%) re-transplantations: the first directly after primary LT due to primary nonfunction, the second at over 2 months after primary transplantation because of hepatic artery thrombosis, and the third after 3 years for de novo hepatitis C virus infection and liver failure.
Because the metastatic tumors in the liver were described as “numerous” or “multiple” in some of the pathological reports, the data on tumors were difficult to analyze. Nevertheless, the information on number of disseminated and countable tumors, their maximal and minimal length, and number for countable tumors are presented in Table 3.
The OS was 94.7%, 88.0%, 88.0%, 70.4%, and 49.3% after 1, 3, 5, 10, and 15 years, respectively, and the RFS was 93.8%, 72.9%, 64.8%, 27.8%, and 27.8% after 1, 3, 5, 10, and 15 years (Figure 2).
Univariable analysis revealed that Ki-67 ≥5% was a risk factor for worse OS – hazard ratio (HR) 7.13 and 95% confidence intervals (95% CI) 1.32–38.63,
Backward elimination left 2 variables (Table 2) in multivariable analysis, which revealed Ki-67 ≥5% was an independent factor for worse OS (HR 13.78, 95% CI 1.48–128.56,
Discussion
Similar to the outcomes of a multicenter study of NELM Eshmuminov et al, the patients in our study were young, with a median age of 47, but most patients had the primary lesions located in the pancreas as opposed to the small bowel [15].
The results presented in the present study are comparable to those reported by other centers. According to the literature, the 5-year OS varies from 33% to 97.2%, and the 5-year RFS ranges from 11% to 86.9% [21,24–29], but results and eligibility criteria differ significantly among centers [30]. The best outcomes are achieved in the Milan center, where the most commonly applied criteria come from [28]. In our group, there were 3 patients beyond the Milan Criteria: one of them was over 60 years old, another had liver involved by neoplasm in over 50%, and the last one exceeded both of these criteria, but the exclusion of those patients did not change our outcomes significantly.
Searching for risk factors, we set the Ki-67 cut-off points as 3%, 5%, and 10%. The strongest effect, defined as the highest HR and the lowest
Older recipient’s age was found to be a risk factor for worse RFS, but is not usually a contraindication for LT [33]. Some studies show it as a predictor of survival following LT for NELM, but there is no consensus on the precise age [22,34,35]. The most commonly accepted is 60 years, which is included in the widely applied Milan Criteria [28]. Because only 2 patients in our study were over 60 years old, we decided to use age 55 years as a cut-off point, similar to the cut-off point previously used in the literature [36]. Age ≥55 years was found to be associated with significantly worse RFS, but not with OS. Importantly, age ≥50 was not significantly associated with worse outcomes, contrary a previous study [34].
In broader context, age is not unequivocally associated with risk of neoplastic disease recurrence. In some types of tumors, younger patients are more susceptible to disease relapse, as neoplasms in that group are often more aggressive [37,38]. Previous studies found no association between age and disease recurrence after primary lesion resection in NEN [39–41], but recipient age under 60–65 is one of eligibility criteria for LT in some centers because it raises concerns about achievable outcome [15]. However, NEN are very rare tumors and these patients tend to have very good long-term survival, so it should be considered a chronic disease. Additionally, tumors cells are evolving, getting more aggressive over time [42]. For instance, in our group, one patient had LT for G2 tumor, and on disease relapse was diagnosed with a G3 lesion. The disease in older patients typically takes more time to develop, which may result in worse RFS.
Patients who met both risk factors for RFS had significantly worse outcomes. However, the HR for such division is lower than for Ki-67 separately, which is a much stronger predictor of survival than age. Therefore, Ki-67 should be treated as a key factor in LT eligibility criteria. Even older patients can benefit from LT as long as the Ki-67 index stays low, as reflected in the Milan Criteria, in which age over 60 is only a relative contraindication for LT for NELM [28].
Some of the tested factors, contrary to our expectations, turned out to be insignificant. The mitotic count is one of the fundamental factors included in the 2019 WHO NEN classification, but some authors report it has no advantage over use of Ki-67 alone [43]. However, the most astonishing example is the insignificance of liver involvement, which is included in the Milan Criteria [28]. In our study, patients had a wide range of liver involvement, from 1% to 60%, but it did not have a significant impact on outcomes.
Because this was a retrospective study, we were unable to retrieve all the data for some patients (missing data are reported in Table 3). Given that only 19 patients were included in this study, the insignificance of tested factors should be treated cautiously. Another limitation is difficulty in uniform assessment and classification of neoplasms. According to the literature, this problem especially affects borderline tumors with Ki-67 values of 2–5% in the commonly used eyeball estimation technique (EE). As a result, true G1 tumors, defined as Ki-67 <3% obtained in digital image analysis (DIA), achieve in EE an average Ki-67 of 4.5±2.0% [44]. Also, there are suggestions that only cells above some intensity of staining should be included in the assessment. That assumption considers Ki-67 protein functions in cells and can change the classification of borderline tumors [45]. Another aspect of Ki-67 assessment is its huge heterogeneity, not only between primary tumors and metastases, but also between simultaneous metastatic lesions and inside a particular lesion [46–48]. The conclusions of previous studies emphasize the complexity of the issue and the problems affecting all research on NET, which may lead to differences in clinical outcomes.
Conclusions
In conclusion, patients with NELM tend to have very good outcomes after LT. The factor associated with worse outcomes is tumor aggressiveness. Ki-67 appears to be a valuable indicator of tumor biology and thus of clinical outcome. Nevertheless, measurement is difficult to objectify, which may lead to discrepancies in clinical outcomes.
Figures
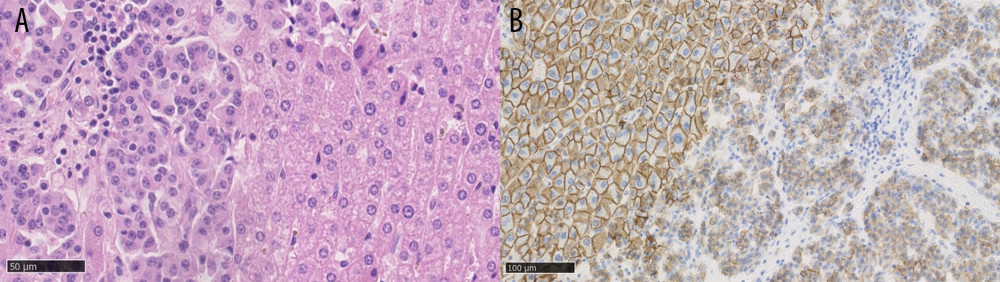 Figure 1. (A) Neuroendocrine liver metastases, hematoxylin and eosin staining, showing a well-differentiated tumor on the left side. (B) Neuroendocrine liver metastases, E-cadherin staining, showing loss of E-cadherin expression in tumor cells on the right side. Created using NDP.view2 (U12388-01), version 2.9.29, Hamamatsu Photonics.
Figure 1. (A) Neuroendocrine liver metastases, hematoxylin and eosin staining, showing a well-differentiated tumor on the left side. (B) Neuroendocrine liver metastases, E-cadherin staining, showing loss of E-cadherin expression in tumor cells on the right side. Created using NDP.view2 (U12388-01), version 2.9.29, Hamamatsu Photonics. 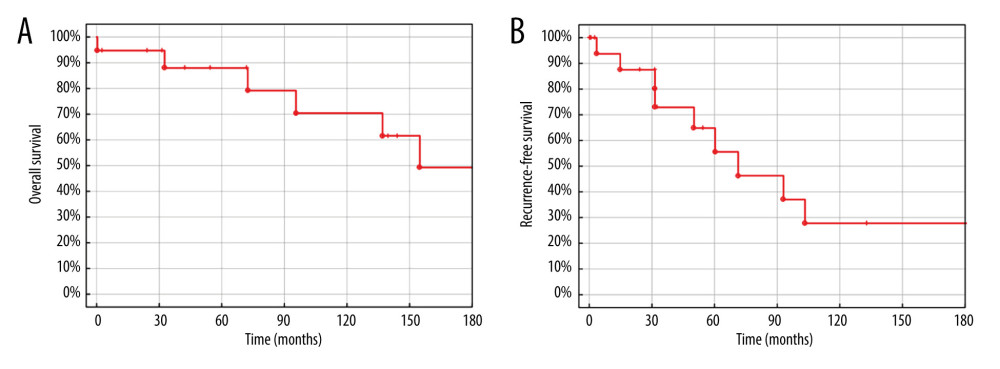 Figure 2. (A) Overall survival of liver transplantation for neuroendocrine liver metastases. (B) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. Created using STATISTICA, version 13.3, StatSoft, Inc., Tulsa, OK, United States.
Figure 2. (A) Overall survival of liver transplantation for neuroendocrine liver metastases. (B) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. Created using STATISTICA, version 13.3, StatSoft, Inc., Tulsa, OK, United States. 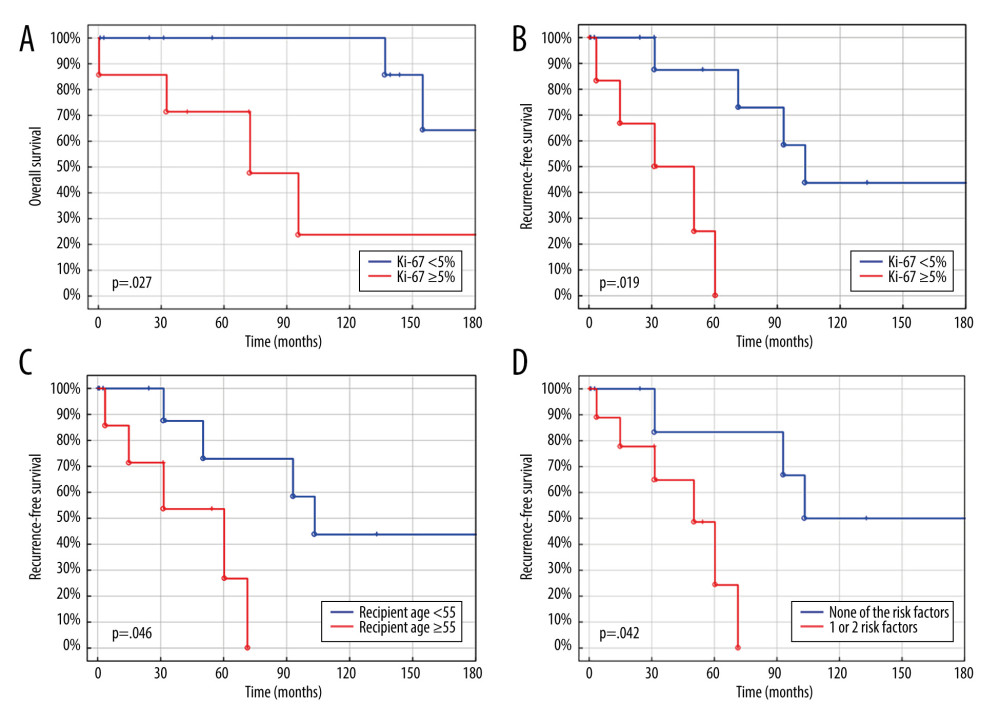 Figure 3. (A) Overall survival of liver transplantation for neuroendocrine liver metastases. (B) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. (C) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. (D) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. Created using STATISTICA, version 13.3, StatSoft, Inc., Tulsa, Oklahoma, United States.
Figure 3. (A) Overall survival of liver transplantation for neuroendocrine liver metastases. (B) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. (C) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. (D) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. Created using STATISTICA, version 13.3, StatSoft, Inc., Tulsa, Oklahoma, United States. References
1. Dasari A, Shen C, Halperin D, Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States: JAMA Oncol, 2017; 3(10); 1335-42
2. Klöppel G, Neuroendocrine neoplasms: Dichotomy, origin and classifications: Visc Med, 2017; 33(5); 324-30
3. Mazzaferro V, Pulvirenti A, Coppa J, Neuroendocrine tumors metastatic to the liver: How to select patients for liver transplantation?: J Hepatol, 2007; 47(4); 460-66
4. Herrera-Martínez AD, Hofland J, Hofland LJ, Targeted systemic treatment of neuroendocrine tumors: Current options and future perspectives: Drugs, 2019; 79(1); 21-42
5. Ngo L, Elnahla A, Attia AS, Chemoembolization versus radioembolization for neuroendocrine liver metastases: A meta-analysis comparing clinical outcomes: Ann Surg Oncol, 2021; 28(4); 1950-58
6. Perrodin SF, Renzulli MM, Maurer MH, can microwave ablation be an alternative to resection for the treatment of neuroendocrine liver metastases?: Endocr Pract, 2020; 26(4); 378-87
7. Machairas N, Daskalakis K, Felekouras E, Currently available treatment options for neuroendocrine liver metastases: Ann Gastroenterol, 2021; 34(2); 130-41
8. Schippers AC, Collettini F, Steffen IG, Initial experience with CT-guided high-dose-rate brachytherapy in the multimodality treatment of neuroendocrine tumor liver metastases: J Vasc Interv Radiol, 2017; 28(5); 672-82
9. Kennedy AS, Dezarn WA, McNeillie P, Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: Early results in 148 patients: Am J Clin Oncol, 2008; 31(3); 271-79
10. Dong XD, Carr BI, Hepatic artery chemoembolization for the treatment of liver metastases from neuroendocrine tumors: A long-term follow-up in 123 patients: Med Oncol, 2011; 28(Suppl 1); S286-90
11. Zener R, Yoon H, Ziv E, Outcomes after transarterial embolization of neuroendocrine tumor liver metastases using spherical particles of different sizes: Cardiovasc Intervent Radiol, 2019; 42(4); 569-76
12. Roche A, Girish BV, de Baere T, Prognostic factors for chemoembolization in liver metastasis from endocrine tumors: Hepatogastroenterology, 2004; 51(60); 1751-56
13. Akyildiz HY, Mitchell J, Milas M, Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: Long-term follow-up: Surgery, 2010; 148(6); 1288-93 discussion 1293
14. Ruzzenente A, Bagante F, Bertuzzo F, Liver resection for neuroendocrine tumor liver metastases within Milan Criteria for liver transplantation: J Gastrointest Surg, 2019; 23(1); 93-100
15. Eshmuminov D, Studer DJ, Lopez Lopez V, Controversy over liver transplantation or resection for neuroendocrine liver metastasis: Tumor biology cuts the deal: Ann Surg, 2022 Online ahead of print
16. Müller PC, Kabacam G, Vibert E, Current status of liver transplantation in Europe: Int J Surg, 2020; 82S; 22-29
17. Lewis A, Koukoura A, Tsianos GI, Organ donation in the US and Europe: The supply vs demand imbalance: Transplant Rev (Orlando), 2021; 35(2); 100585
18. D’Amico G, Uso TD, Del Prete L, Neuroendocrine liver metastases: The role of liver transplantation: Transplant Rev (Orlando), 2021; 35(2); 100595
19. Tsoli M, Chatzellis E, Koumarianou A, Current best practice in the management of neuroendocrine tumors: Ther Adv Endocrinol Metab, 2018; 10; 2042018818804698
20. Grąt M, Remiszewski P, Smoter P, Outcomes following liver transplantation for metastatic neuroendocrine tumors: Transplant Proc, 2014; 46(8); 2766-69
21. Olausson M, Friman S, Herlenius G, Orthotopic liver or multivisceral transplantation as treatment of metastatic neuroendocrine tumors: Liver Transpl, 2007; 13(3); 327-33
22. Le Treut YP, Grégoire E, Klempnauer Jfor ELITA, Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: A 213-case European liver transplant registry study: Ann Surg, 2013; 257(5); 807-15
23. Rosenau J, Bahr MJ, von Wasielewski R, Ki67, E-cadherin, and p53 as prognostic indicators of long-term outcome after liver transplantation for metastatic neuroendocrine tumors: Transplantation, 2002; 73(3); 386-94
24. van Vilsteren FG, Baskin-Bey ES, Nagorney DM, Liver transplantation for gastroenteropancreatic neuroendocrine cancers: Defining selection criteria to improve survival: Liver Transpl, 2006; 12(3); 448-56
25. Frilling A, Malago M, Weber F, Liver transplantation for patients with metastatic endocrine tumors: Single-center experience with 15 patients: Liver Transpl, 2006; 12(7); 1089-96
26. Korda D, Doros A, Piros L, Liver transplant for metastatic neuroendocrine tumors: A single-center experience in hungary: Transplant Proc, 2019; 51(4); 1251-53
27. Nguyen NT, Harring TR, Goss JA, O’Mahony CA, Neuroendocrine liver metastases and orthotopic liver transplantation: The US experience: Int J Hepatol, 2011; 2011; 742890
28. Mazzaferro V, Sposito C, Coppa J, The long-term benefit of liver transplantation for hepatic metastases from neuroendocrine tumors: Am J Transplant, 2016; 16(10); 2892-902
29. Bonaccorsi-Riani E, Apestegui C, Jouret-Mourin A, Liver transplantation and neuroendocrine tumors: Lessons from a single centre experience and from the literature review: Transpl Int, 2010; 23(7); 668-78
30. Fan ST, Le Treut YP, Mazzaferro V, Liver transplantation for neuroendocrine tumour liver metastases: HPB (Oxford), 2015; 17(1); 23-28
31. Nagtegaal ID, Odze RD, Klimstra DWHO Classification of Tumours Editorial Board, The 2019 WHO classification of tumours of the digestive system: Histopathology, 2020; 76(2); 182-88
32. Scarpa A, Mantovani W, Capelli P, Pancreatic endocrine tumors: Improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients: Mod Pathol, 2010; 23(6); 824-33
33. Dolnikov S, Adam R, Cherqui D, Allard MA, Liver transplantation in elderly patients: What do we know at the beginning of 2020?: Surg Today, 2020; 50(6); 533-39
34. Lehnert T, Liver transplantation for metastatic neuroendocrine carcinoma: An analysis of 103 patients: Transplantation, 1998; 66(10); 1307-12
35. Kim J, Zimmerman MA, Hong JC, Liver transplantation in the treatment of unresectable hepatic metastasis from neuroendocrine tumors: J Gastrointest Oncol, 2020; 11(3); 601-8
36. Pavel M, Baudin E, Couvelard ABarcelona Consensus Conference participants, ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary: Neuroendocrinology, 2012; 95(2); 157-76
37. Ali P, Fonseca C, Heidenreich A, Ruthberg R, Damadi A, Is colorectal neoplasia more aggressive in the young? A look at shifting guidelines: Am J Surg, 2022; 223(3); 496-98
38. Xie X, Yin J, Zhou Z, Young age increases the risk for lymph node metastasis in patients with early Colon Cancer: BMC Cancer, 2019; 19(1); 803
39. Casadei R, Ricci C, Pezzilli R, Are there prognostic factors related to recurrence in pancreatic endocrine tumors?: Pancreatology, 2010; 10(1); 33-38
40. Kim DH, Lee JH, Cha YJ, Surveillance strategy for rectal neuroendocrine tumors according to recurrence risk stratification: Dig Dis Sci, 2014; 59(4); 850-56
41. Shen C, Dasari A, Chu Y, Clinical, pathological, and demographic factors associated with development of recurrences after surgical resection in elderly patients with neuroendocrine tumors: Ann Oncol, 2017; 28(7); 1582-89 Erratum in: Ann Oncol. 2019;30(11):1847
42. Keck KJ, Choi A, Maxwell JE, Increased grade in neuroendocrine tumor metastases negatively impacts survival: Ann Surg Oncol, 2017; 24(8); 2206-12
43. Khan MS, Luong TV, Watkins J, A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms: Br J Cancer, 2013; 108(9); 1838-45
44. Tang LH, Gonen M, Hedvat C, Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: A comparison of digital image analysis with manual methods: Am J Surg Pathol, 2012; 36(12); 1761-70
45. Faviana P, Boldrini L, Gentile C, Proposal for a new diagnostic histopathological approach in the evaluation of Ki-67 in GEP-NETs: Diagnostics (Basel), 2022; 12(8); 1960
46. Adesoye T, Daleo MA, Loeffler AG, Discordance of histologic grade between primary and metastatic neuroendocrine carcinomas: Ann Surg Oncol, 2015; 22(Suppl 3); S817-21
47. Shi H, Jiang C, Zhang Q, Clinicopathological heterogeneity between primary and metastatic sites of gastroenteropancreatic neuroendocrine neoplasm: Diagn Pathol, 2020; 15(1); 108
48. Yang Z, Tang LH, Klimstra DS, Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: Implications for prognostic stratification: Am J Surg Pathol, 2011; 35(6); 853-60
Figures
 Figure 1. (A) Neuroendocrine liver metastases, hematoxylin and eosin staining, showing a well-differentiated tumor on the left side. (B) Neuroendocrine liver metastases, E-cadherin staining, showing loss of E-cadherin expression in tumor cells on the right side. Created using NDP.view2 (U12388-01), version 2.9.29, Hamamatsu Photonics.
Figure 1. (A) Neuroendocrine liver metastases, hematoxylin and eosin staining, showing a well-differentiated tumor on the left side. (B) Neuroendocrine liver metastases, E-cadherin staining, showing loss of E-cadherin expression in tumor cells on the right side. Created using NDP.view2 (U12388-01), version 2.9.29, Hamamatsu Photonics. Figure 2. (A) Overall survival of liver transplantation for neuroendocrine liver metastases. (B) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. Created using STATISTICA, version 13.3, StatSoft, Inc., Tulsa, OK, United States.
Figure 2. (A) Overall survival of liver transplantation for neuroendocrine liver metastases. (B) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. Created using STATISTICA, version 13.3, StatSoft, Inc., Tulsa, OK, United States. Figure 3. (A) Overall survival of liver transplantation for neuroendocrine liver metastases. (B) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. (C) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. (D) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. Created using STATISTICA, version 13.3, StatSoft, Inc., Tulsa, Oklahoma, United States.
Figure 3. (A) Overall survival of liver transplantation for neuroendocrine liver metastases. (B) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. (C) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. (D) Recurrence-free survival of liver transplantation for neuroendocrine liver metastases. Created using STATISTICA, version 13.3, StatSoft, Inc., Tulsa, Oklahoma, United States. In Press
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860