13 February 2024: Original Paper
A Retrospective Study of Long-Term Outcomes in 16 ABO-Incompatible Deceased Donor Pediatric Liver Transplants from a National Transplant Center at Helsinki University Hospital, Finland, 1987–2022
Timo JahnukainenDOI: 10.12659/AOT.941929
Ann Transplant 2024; 29:e941929
Abstract
BACKGROUND: The use of ABO-incompatible liver transplants (ABO-ILTs) from deceased donors has become more common due to the shortage of available donor livers and increased transplant waiting times. This retrospective study from a national transplant center at Helsinki University Hospital, Finland, aimed to assess the long-term outcomes of ABO-incompatible deceased donor pediatric liver transplants between 1987 and 2022.
MATERIAL AND METHODS: Sixteen (9.5%) of the 169 pediatric liver transplantations were ABO-ILTs. The median age at transplantation was 5.0 (0.5-15.4) years. Reasons for ABO-ILTs were acute liver failure (18.75%), malignancy (12.5%), small body size and long waiting time (25%), and other reasons (43.75%). The median post-transplant follow-up time was 147 (0.72-353) months. Patient and graft survival and occurrence of surgical complications were compared to ABO-identical transplants, and anti-ABO antibody titers were analyzed.
RESULTS: The 1-, 3-, and 5-year patient survivals were comparable between the ABO-I and ABO-compatible groups, being 81.3%, 73.9%, and 73.9% (ABO-I) and 87.5%, 82.5%, 77.9% (ABO-compatible), respectively. Three patients with ABO-ILTs died of sepsis and multiorgan failure during the first 3 months after transplantation. The occurrence of biliary complications and early vascular thrombosis (<30 days after transplantation) did not differ significantly between recipients with an ABO-ILT vs ABO-compatible liver graft.
CONCLUSIONS: The findings from this study support findings from previous studies that outcomes after ABO-incompatible liver transplants in children were comparable to outcomes from ABO-identical liver transplants.
Keywords: Blood Group Incompatibility, Child, Liver Transplantation, Treatment Outcome
Background
Pediatric liver transplantation (LT) has been a life-saving treatment modality for more than 3 decades [1,2]. The leading indications for liver transplantation in children are cholestatic conditions, such as biliary atresia, metabolic diseases, tumors, and acute liver failure [1–3]. Despite an increased number of split-liver transplantations and possibilities to use living liver donors, a shortage of suitable grafts is an important limiting factor for pediatric LTs [4].
Traditionally, liver grafts are allocated according to blood group compatibility [3]. The development of immunosuppressive drugs in recent years has allowed the old rule of not crossing the ABO blood group to be overturned [5]. In many centers, selected pediatric patients, typically infants below 2 years of age, are considered for organs from mismatch blood group donors due to the difficulty in finding a size-matched organ for recipients in urgent need [3]. ABO-incompatible (ABO-I) liver transplantations have become an acceptable treatment modality in both pediatric and adult patients, accounting for 1–20% of all LT performed in recent decades [6,7].
Based on many small observational studies, ABO-I LT recipients have lower survival rates and higher rates of post-transplant vascular and biliary complications [5,8,9]. However, an increasing number of registry data on ABO-I LT show favorable outcomes for patients receiving an ABO-I liver graft [7,9–13]. Recent register-based studies and meta-analyses have reported nearly comparable patient and graft survival rates between ABO-I and ABO-compatible (ABO-C) LT [5,6,11]. Furthermore, the occurrence of complications such as vascular thrombosis and biliary stenosis is not increased among recipients with ABO-I grafts compared to those with ABO-C transplants [5,10,12,14,15].
In Finland, the first pediatric ABO-I LT was performed in the late 1980s. Since then, 16 (10.5%) patients have received an ABO-incompatible liver graft. In this retrospective nation-wide single-center study, we report the results of all pediatric ABO-I LTs performed at our center from 1987 to 2022. We collected data on pre-existing and post-transplant ABO antibody titers and the prevalence of vascular and biliary complications in relation to the general complication rate at our unit. We hypothesized that patients who survive the first post-transplant months would have outcomes comparable to those of ABO-C LT recipients. Therefore, this study from a national transplant center at Helsinki University Hospital, Finland, aimed to evaluate the long-term outcomes of the 16 pediatric ABO-I deceased donor liver transplants between 1987 and 2022.
Material and Methods
ETHICS APPROVAL:
The study was performed at the Children’s Hospital, Helsinki University Hospital. The Research Ethics Committee of Helsinki and Uusimaa Hospital District approved the study as part of the research project entitled “Development of Pediatric Solid Organ Transplantation Program” (HUS 939/2018). The study was a retrospective register-based analysis and according to current regulations, no informed written consent from study subjects or their caregivers are required.
The authors declare no conflicts of interest.
PATIENTS AND DATA COLLECTION:
The present study is a single-center retrospective study consisting of all pediatric LT recipients who received an ABO-I liver graft from a deceased donor between 1987 and 2022 in Finland. All pediatric (age ≤16 years) solid organ transplantations (SOT) are centralized to the Children’s Hospital, Helsinki University Hospital, and all pediatric SOT recipients are followed up at our institution at least once yearly until the age of 18 to 20 years.
A total of 169 LT was performed on 144 patients by the end of the year 2022, and 16 patients with ABO-I grafts were identified. The data on pre-existing and post-transplantation ABO and HLA antibodies, surgical complications, immunosuppression, and graft and patient survivals were collected from hospital records when available.
ABO ANTIBODY TITER ANALYSIS:
ABO antibodies were analyzed from stored serum samples from patients whose antibody result was missing and whose serum sample was available. Anti-A and/or anti-B titers were measured with column agglutination technique (CAT) without dithiothreitol. The manual method was used because of the low sample volume. IgM was measured with direct agglutination and IgG with antiglobulin technique according to the Bio-Rad procedure. Titers were reported of the concentration in the last 1+ agglutination. Exclusion of irregular Rh and non-Rh antibodies was done with CAT by IH-500 automation.
IMMUNOSUPPRESSION PROTOCOL:
Until the year 2000, neither monoclonal nor polyclonal antibodies were included in our standard induction immunosuppression protocol. Since the year 2000, basiliximab has been used routinely as induction therapy with few exceptions. The maintenance immunosuppression protocol of LT recipients consisted of triple medication. The most commonly used drug combination after LT was cyclosporine A (CsA), azathioprine (AZA), and methylprednisolone (MP) until the year 2010, after which tacrolimus (Tac) with AZA or mycophenolate mofetil (MMF) has been the first-choice immunosuppression. Initially, MP was dosed daily; later, the regimen was switched to alternate-day dosing at 3–6 months after LT.
STATISTICAL ANALYSIS:
Numerical data are presented as medians with ranges. The Mann-Whitney U test was used to compare key surgical parameters and the chi-square test was performed to compare the occurrence of surgical complications between the patients with ABO-incompatible and compatible grafts. Findings were considered statistically significant when the probability of a chance finding was less than 5% (
Results
PATIENT DEMOGRAPHICS:
The key patient characteristics are presented in Table 1. The median age at transplantation of all pediatric LT patients was 3.8 (0.4–16.3) years. The pre-transplant diagnoses comprised biliary atresia (39%), metabolic disease (22%), malignancy (14%), acute liver failure (11%), and miscellaneous (14%). The re-transplantation rate was 15.7%.
Recipients with ABO-I LT had a median age of 5.04 (0.52–15.4) years at LT and the median post-LT follow-up time was 147 (0.72–353) months. The reasons for ABO-incompatible LT were acute liver failure (18.75%), tumor (12.5%), small body size and long waiting time (25%), and other reasons (43.75%) (Table 1).
POST-LT IMMUNOSUPPRESSION:
Most patients with an ABO-I graft received either antithymocyte globulin (ATG) or basiliximab induction therapy (Table 2). Therapeutic plasma exchange (TPE) was performed in 2 patients before LT and in 3 patients after the operation. The need for TPE was decided on a case-by-case basis depending on the ABO titers. Half of the patients had CsA, AZA, and MP as primary maintenance immunosuppression and the other half had Tac combined with either AZA or MMF (Table 2). The choice of calcineurin inhibitor was influenced more by the transplant era than by ABO compatibility.
ANTI-ABO ANTIBODY TITERS:
The majority (75%) of the recipients receiving an ABO-I liver graft had blood group O, while 56.3% of the donors had ABO blood group A (Table 3). The information about the A subtype was not available. The highest detected pre- and post-transplant ABO IgG titer was 1: 32 and 1: 256, respectively (Table 3). The patient with the highest post-transplant ABO antibody titer had a pre-transplant ABO antibody titer of 1: 4, but after an acute hepatic artery thrombosis she developed a high anti-B antibody titer. She lost her graft despite MP pulse therapy, ATG, and TPE.
PATIENT AND GRFT SURVIVAL:
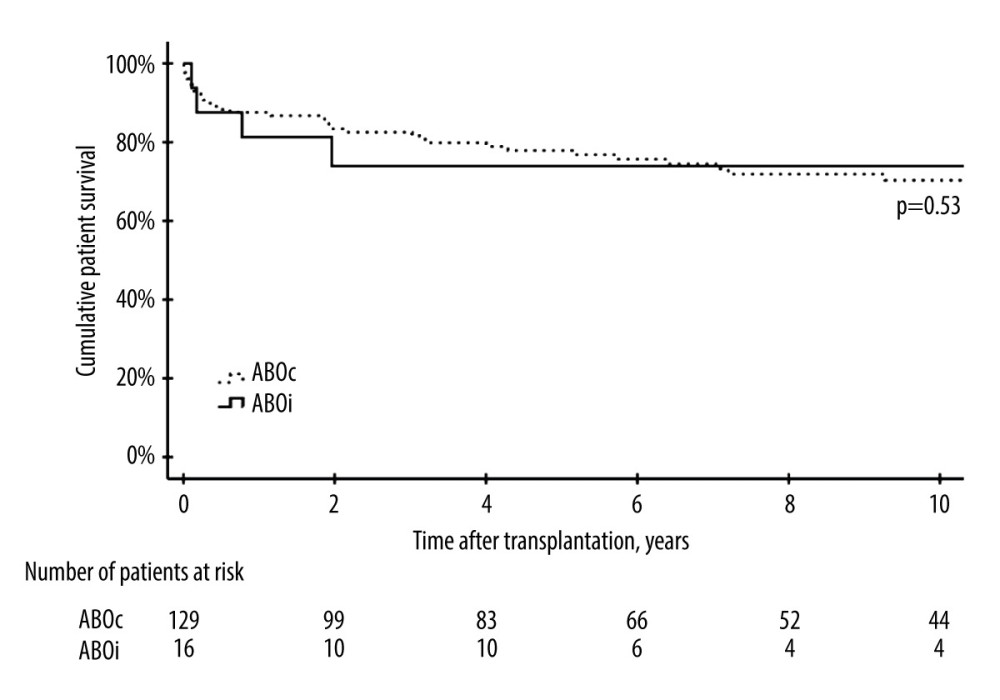
The 1-, 3-, and 5-year patient survivals were comparable between the ABO-incompatible and ABO-compatible groups, being 81.3%, 73.9%, and 73.9% (ABO-I) and 87.5%, 82.5%, 77.9% (ABO-C), respectively. Six patients (37.5%) died in the ABO-incompatible group and 33 (25.8%) died in the ABO-compatible group (P=0.133) (Figure 1). In the ABO-incompatible group, the median age at death was 7.86 (0.1–24.1) years and the causes of death included progression of the primary disease (Farber’s disease) (n=1), post-transplantation lymphoproliferative disease (PTLD) (n=1), anthracycline-induced heart failure (n=1), and sepsis with multiorgan failure (n=3). All patients with sepsis died during the first 3 months after transplantation. One patient (6.3%) in the ABO-incompatible group experienced graft loss, while 24 (15.7%) re-transplantations were performed in the ABO-C group (P=0.312).
Five (31%) patients with an ABO-I graft had mild cellular allograft rejection; 4 of them were detected within the first 3 post-LT months. Three patients (18.8%) developed Cytomegalovirus viremia within the first 30 days after LT despite antiviral prophylaxis. Two patients (12.5%) developed Epstein-Barr virus-positive PTLD 2 and 3 years after the LT.
VASCULAR AND BILIARY COMPLICATIONS:
Hepatic artery stenosis or thrombosis was detected in 5 (31.3%) patients and portal vein thrombosis or stenosis was found in 2 patients (12.5%) in the ABO-I group (Table 4). The prevalence of early vascular complications appearing during the first 30 post-transplantation days did not differ statistically significantly between the patients with an ABO-I and ABO-C liver graft (Table 4). In 2 cases, hepatic artery thrombosis was detected during the first 4 weeks after the ABO-I LT. One patient was treated successfully with streptomycin. The other patient lost her graft and was retransplanted successfully a few months after the first LT. Three additional patients (18.8%) experienced late hepatic artery thrombosis (HAT) (>30 days after the ABO-I LT). In 1 patient, the HAT was detected approximately 2 months after the LT. The patient had developed collateral arterial circulation and no special treatment was required. Two patients had a late HAT 1 and 9 years after the transplantation. The latter patient developed vanishing bile duct syndrome, but there was no need for re-LT.
The patients with vascular complications tended to be younger at LT than the ones without any recorded complication, 1.08 (0.52–3.13) years vs 3.82 (0.69–15.34) years, respectively (
Stenosis of the biliary anastomosis was found in 1 patient approximately 18 months after LT (Table 4). This same patient had hepatic artery stenosis and was treated successfully with percutaneous transhepatic drainage.
Discussion
The published data on patient and graft survival and risk for post-transplant complications after pediatric ABO-incompatible LT have been divergent [12,16,17]. The outcomes likely depend on the age and size of the recipient and reason and urgency for LT [14]. According to current literature, infants younger than 2 years of age have the best outcomes after ABO-I LT [12,17]. In our cohort, consisting of 16 children with a median age of 5.0 years, the outcome after deceased donor pediatric recipients did not differ between ABO-I and ABO-C LTs, the 1-, 3-, and 5-year patient survivals being 81.3%, 73.9%, and 73.9% (ABO-I) and 87.5%, 82.5%, 77.9% (ABO-C), respectively. The incidence of vascular and biliary complications was similar between the 2 groups. Our findings agree with the recent large European study by Markiewicz-Kijewska et al, which showed no significant difference in the outcomes of infants and older children after ABO-I LT [5]. This supports the feasibility of this treatment modality in a selected pediatric population needing LT.
The first studies reported inferior outcomes after ABO-I LTs [6]. It is likely that at least in the beginning of the ABO-I LT program, selection for ABO-I LT has favored patients with urgent need for LT, long waiting time, and small body size, which are known risk factors for postoperative complications and mortality. It is also likely that patients with ABO-I LT tend to receive more intense immunosuppression, which increases the risk for post-transplant infections and PTLD, especially in pediatric patients. Based on our relatively small patient cohort, the long-term outcome of ABO-I LT recipients did not differ from that of patients with ABO-C grafts. Three of the patients died within the first 3 post-transplant months due to sepsis and multiorgan failure and 2 of these patients received ATG as induction therapy, which may have increased their susceptibility for post-transplant infections. ATG has not been used in our center after 1993, and since then none of the ABO-I LT recipients have died of septicemia. Other deaths in our ABO-I LT cohort were unlikely to be related to blood group incompatibility.
It has been suggested that ABO isoagglutinins targeting the bile duct epithelium and vascular endothelium of the graft, can lead to thrombosis and finally, ischemic injury. This result in rapid graft loss post-transplant or gradual graft failure months later [17]. About 30% of our ABO-I cohort experienced either hepatic artery or portal vein stenosis or thrombosis, and 1 patient had biliary tract stenosis. The frequency of vascular complications was relatively high compared to previous literature [1,10,12]; however, early hepatic artery thrombosis (HAT) occurred in 12.5% of the ABO-incompatible group, 4.5% of the ABO-compatible group, and 8.5% in the whole cohort, consistent with generally reported rates [5,8,9,13]. In our cohort, the measured anti-ABO titers before or after LT did not seem to influence hepatic artery or portal vein events. Notably, patients had a relatively low pre-transplant anti-ABO antibody titer (≤1: 32). Since 85% of the patients with HAT were younger than 2 years of age at LT and only 3 vascular complications have been found during the last 20 years, the risk for thrombosis in our cohort may have been more affected by factors other than ABO blood group compatibility.
Late hepatic artery thrombosis was observed in 18.8% of our ABO-I cohort. Our prior cross-sectional cohort study indicated that late hepatic artery thrombosis occurs in approximately 40% of patients several years after LT and that ultrasonography’s sensitivity in detecting arterial thrombi is relatively low [18]. Hence, assessing the impact of ABO compatibility on late vascular complications may be challenging.
The early unsuccessful attempts to cross the ABO barrier clearly showed that the presence of anti-ABO antibodies is important. For this reason, several protocols, including splenectomy, TPE, immunoadsorption, high-dose intravenous immunoglobulins, or antiCD20 monoclonal antibodies, have been developed to prevent humoral rejection and to improve graft survival. However, whether additional immunosuppression is needed remains unclear [17,19–21]. There are studies showing correlations between the anti-ABO titer and post-transplant complications [22] while others, including the present study, have suggested that pre- or post-transplant ABO titers do not affect graft survival [5,19,23,24]. There may also be inter-individual variation in the development of immunological tolerance, which is likely to influence long-term outcome after ABO-I transplantation. In our cohort, approximately 30% of the patients presented with mild biopsy-proven rejection and none with verified humoral rejection. This finding is in accordance with a recent European multicenter study [5] and suggests that patients with low level of anti-ABO antibodies can be treated using standard immunosuppression.
A limitation of our study is its retrospective nature and the small number of patients. Additionally, anti-ABO antibody titers were not available from all patients, but our 68.8% collection rate was relatively robust compared to most previous studies. The strength of our study is that it represents a national cohort transplanted and followed up without dropouts. The follow-up time was long, with a median of 12.2 years.
Conclusions
In conclusion, the findings from this study support findings from previous studies that outcomes after ABO-incompatible liver transplants in children were comparable to outcomes from ABO-identical liver transplants.
Tables
Table 1. Patient demographics describing a cohort of 16 pediatric patients who received an ABO-incompatible liver transplant in Finland. Table 2. Use of therapeutic plasma exchange before and after liver transplantation and immunosuppression regimens in pediatric liver transplant patients with an ABO-incompatible liver transplant in Finland.
Table 2. Use of therapeutic plasma exchange before and after liver transplantation and immunosuppression regimens in pediatric liver transplant patients with an ABO-incompatible liver transplant in Finland.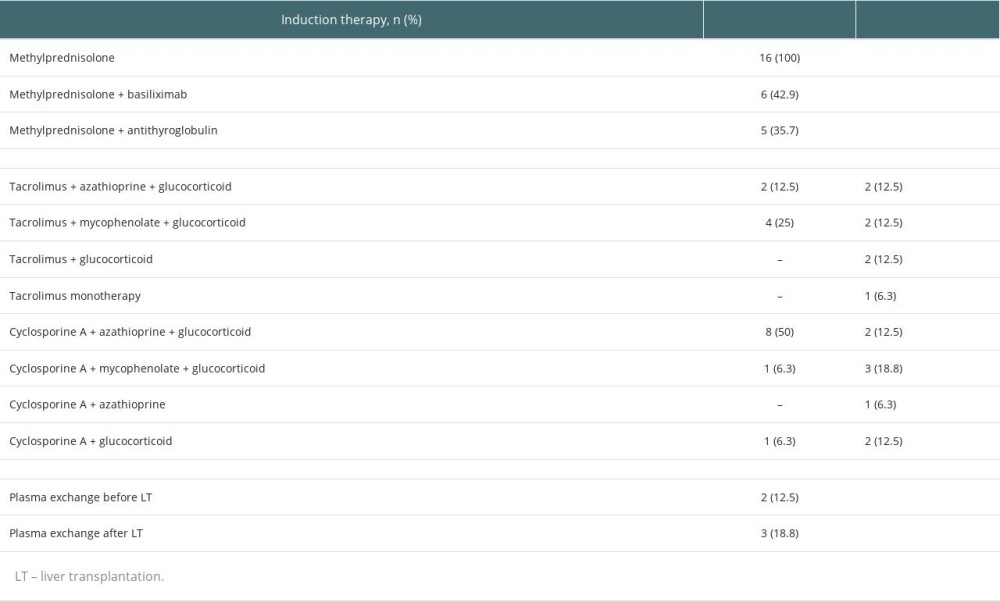 Table 3. Sensitization against HLA antigens and anti-ABO antibodies before and after liver transplantation among pediatric patients who have received an ABO-incompatible liver transplant in Finland.
Table 3. Sensitization against HLA antigens and anti-ABO antibodies before and after liver transplantation among pediatric patients who have received an ABO-incompatible liver transplant in Finland.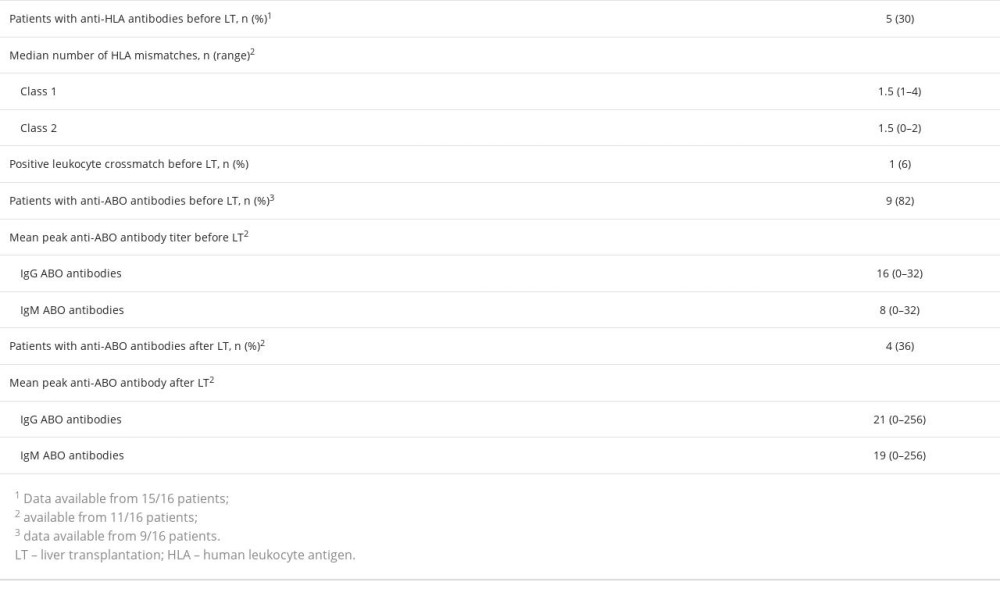 Table 4. Comparison of the surgical data between 16 pediatric ABO-incompatible and 153 ABO-compatible pediatric liver transplantations in Finland.
Table 4. Comparison of the surgical data between 16 pediatric ABO-incompatible and 153 ABO-compatible pediatric liver transplantations in Finland.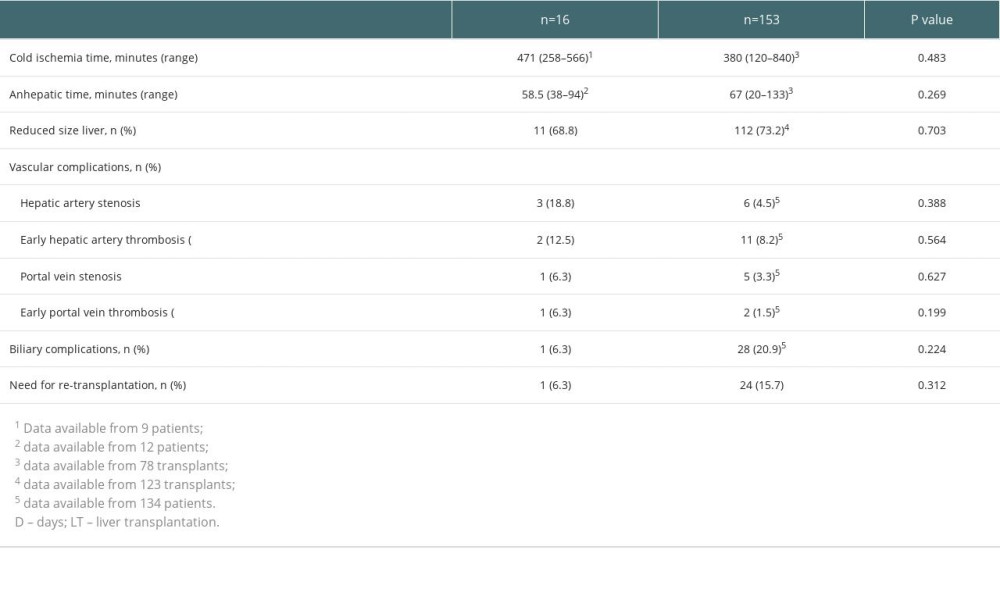
References
1. de Ville de Goyet J, Bumann U, Karam Vfor the European Liver, Intestine Transplant Association. European Liver Transplant Registry, Donor and transplant surgery aspects of 16,641 liver transplantations in children: Hepatology, 2022; 75; 634-45
2. Dababneh Y, Mousa OY, Liver transplantation. [Updated 2023 Apr 7]: StatPearls [Internet] Jan, 2023, Treasure Island (FL), StatPearls Publishing Available fromhttps://www.nbci.nlm.nih.gov/books/NBK559161/
3. Kohli R, Cortes M, Heaton ND, Dhawan A, Liver transplantation in children: State of the art and future perspectives: Arch Dis Child, 2018; 103; 192-98
4. Kim WR, Lake JR, Smith JM, OPTN/SRTR 2017 Annual data report: Liver Am J Transplant, 2019(Suppl 2); 184-283
5. Markiewicz-Kijewska M, Kalicinski P, Canizales JTon behalf of ERN TransplantChild Healthcare Working Group, ABO incompatible liver transplantation in children: A 20 year experience from Centres in the TransplantChild European Reference Network: Children, 2021; 8; 760
6. Farges O, Kalil AN, Samuel D, The use of ABO-incompatible grafts in liver transplantation: A life-saving procedure in highly selected patients: Transplantation, 1995; 59; 1124-33
7. Lai JC, Roberts JP, ABO-nonidentical liver transplantation in the United States: Am J Transplant, 2016; 16; 2430-36
8. Kang Z-Y, Liu W, Li D-H, Comparison of clinical outcomes between ABO-incompatible and ABO-compatible pediatric liver transplantation: A systematic literature review and meta-analysis: Pediatr Surg Int, 2020; 36; 1353-62
9. Lee EC, Kim SH, Park S-J, Outcomes after liver transplantation in accordance with ABO compatibility: A systematic review and meta-analysis: World J Gastroenterol, 2017; 23; 6516-33
10. Gan K, Li Z, Bao S, Clinical outcomes after ABO-incompatible liver transplantation: A systematic review and meta-analysis: Transplant Immunol, 2021; 69; 101476
11. Sun C, Song Z, Ma N, The management and outcomes of ABO-incompatible pediatric liver transplantation: Experience of a single Chinese center: J Ped Surg, 2020; 55; 2647-52
12. Wu J, Ye SY, Xu XF, Recipient outcomes after ABO-incompatible liver transplantation: A systematic review and meta-analysis: PLoS One, 2021; 6; e16521
13. Prachuapthunyachart S, Sintusek P, Tubjareon C, Pediatric liver transplantation outcomes from a single center in Thailand: World J Hepatol, 2022; 27; 583-91
14. Kim SH, Park J, Park SJ, Impact of ABO-incompatibility on hepatic artery thrombosis in living donor liver transplantation: Ann Transpl Med, 2019; 7; 625
15. Lemoine CP, Brandt KA, Keswani M, Superina R, Outcomes after ABO incompatible pediatric liver transplantation are comparable to ABO identical/compatible transplant: Front Pediatr, 2023; 11; 1092412
16. Elisofon SA, Magee JC, Ng VLfor the Society of Pediatric Liver Transplantation Research Group, Society of pediatric liver transplantation: Current registry status 2011 2018: Pediatr Transpl, 2019; 24; e13605
17. Kasahara M, Umeshita K, Sakamoto Sthe Japanese Liver Transplantation Society, Living donor liver transplantation for biliary atresia: An analysis of 2085 cases in the registry of the Japanese Liver Transplantation Society: Am J Transplant, 2018; 18; 659-68
18. Kivelä JM, Kosola S, Kalajoki-Helmiö T, Late hepatic artery thrombosis after pediatric liver transplantation: A cross-sectional study of 34 patients: Liver Transplant, 2014; 20; 591-600
19. Song G-W, Lee S-G, Hwang S, Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era: J Hepatol, 2014; 61; 575-82
20. Honda M, Sugawara Y, Kadohisa M, Long-term outcomes of ABO-incompatible pediatric living donor liver transplantation: Transplantation, 2018; 102; 1702-9
21. Mysore KR, Himes RW, Rana A, ABO-incompatible deceased donor pediatric liver transplantation: Novel titer-based management protocol and outcomes: Pediatr Transpl, 2018; 22; e13263
22. Ueda D, Yoshizawa A, Kaneshiro M, Low titers of antidonor ABO antibodies after ABO-incompatible living donor liver transplantation: A long-term follow-up study: Transplantation Direct, 2018; 4; e420
23. Kim SH, Lee EC, Shim JR, Park SJ, A simplified protocol using rituximab and immunoglobulin for ABO-incompatible low-titre living donor liver transplantation: Liver Int, 2018; 38; 932-39
24. Skogsberg U, Breimer ME, Friman S, Adult ABO-incompatible liver transplantation, using A2 and B donors: Xenotransplantation, 2006; 13; 154-59
Tables
 Table 1. Patient demographics describing a cohort of 16 pediatric patients who received an ABO-incompatible liver transplant in Finland.
Table 1. Patient demographics describing a cohort of 16 pediatric patients who received an ABO-incompatible liver transplant in Finland. Table 2. Use of therapeutic plasma exchange before and after liver transplantation and immunosuppression regimens in pediatric liver transplant patients with an ABO-incompatible liver transplant in Finland.
Table 2. Use of therapeutic plasma exchange before and after liver transplantation and immunosuppression regimens in pediatric liver transplant patients with an ABO-incompatible liver transplant in Finland. Table 3. Sensitization against HLA antigens and anti-ABO antibodies before and after liver transplantation among pediatric patients who have received an ABO-incompatible liver transplant in Finland.
Table 3. Sensitization against HLA antigens and anti-ABO antibodies before and after liver transplantation among pediatric patients who have received an ABO-incompatible liver transplant in Finland. Table 4. Comparison of the surgical data between 16 pediatric ABO-incompatible and 153 ABO-compatible pediatric liver transplantations in Finland.
Table 4. Comparison of the surgical data between 16 pediatric ABO-incompatible and 153 ABO-compatible pediatric liver transplantations in Finland. Table 1. Patient demographics describing a cohort of 16 pediatric patients who received an ABO-incompatible liver transplant in Finland.
Table 1. Patient demographics describing a cohort of 16 pediatric patients who received an ABO-incompatible liver transplant in Finland. Table 2. Use of therapeutic plasma exchange before and after liver transplantation and immunosuppression regimens in pediatric liver transplant patients with an ABO-incompatible liver transplant in Finland.
Table 2. Use of therapeutic plasma exchange before and after liver transplantation and immunosuppression regimens in pediatric liver transplant patients with an ABO-incompatible liver transplant in Finland. Table 3. Sensitization against HLA antigens and anti-ABO antibodies before and after liver transplantation among pediatric patients who have received an ABO-incompatible liver transplant in Finland.
Table 3. Sensitization against HLA antigens and anti-ABO antibodies before and after liver transplantation among pediatric patients who have received an ABO-incompatible liver transplant in Finland. Table 4. Comparison of the surgical data between 16 pediatric ABO-incompatible and 153 ABO-compatible pediatric liver transplantations in Finland.
Table 4. Comparison of the surgical data between 16 pediatric ABO-incompatible and 153 ABO-compatible pediatric liver transplantations in Finland. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860