13 November 2020: Original Paper
Single-Center Retrospective Analysis of Prophylaxis and Treatment of Pneumonia in Patients with Renal Dysfunction After Renal Transplantation
Jianyong Pan1C, Yingxin Fu1A*, Yu Cao1C, Gang Feng1F, Jie Zhao1D, Xiaofeng Shi1D, Chunbai Mo1A, Wenli Song1F, Zhongyang Shen1AGDOI: 10.12659/AOT.925126
Ann Transplant 2020; 25:e925126
Abstract
BACKGROUND: Pneumocystis carinii is an opportunistic pathogen that can cause severe lung infections after renal transplantation. Trimethoprim-sulfamethoxazole (TMP-SMX) has been recognized as a first-line treatment for chemoprophylaxis of Pneumocystis carinii pneumonia (PCP). This study aimed to establish a personalized chemoprophylaxis prescription specifically for those recipients with renal insufficiency.
MATERIAL AND METHODS: This retrospective study included 68 patients with confirmed PCP after renal transplantation. Patients were divided into 2 groups: an abnormal renal function (ARF) group (creatinine ≥1.5 ng/dl; n=37) and a normal renal function (NRF) group (creatinine <1.5 ng/dl; n=31). Clinical characteristics and prognosis of PCP in both groups were compared and analyzed.
RESULTS: Patients in the ARF group had more prophylaxis after transplantation (15 [40.5%] vs. 2 [6.5%], p=0.047), had more biopsy-proven rejections (10 [27%] vs. 1 [3.2%], p=0.008), and had lower lymphocyte counts (0.6 [05–0.9] vs. 1.1 [0.7–1.6], p<0.01). Renal function after treatment was obviously improved in the ARF group, which had a significant decrease rate in creatinine (–13.2% [–22~4.8%] vs. –4.4% [–12.6~20.9%], p=0.043).
CONCLUSIONS: PCP prophylaxis regimens for recipients after renal transplantation are still needed regardless of whether the renal functions were normal or abnormal, especially for recipients with persistent lymphopenia or rejection after transplantation.
Keywords: Antibiotic Prophylaxis, Kidney Transplantation, Pneumonia, Pneumocystis, Kidney Diseases, Pneumocystis carinii
Background
In renal transplant recipients,
Material and Methods
STUDY POPULATION:
This retrospective control study included 68 patients with confirmed PCP after renal transplantation between March 2011 and October 2017 at our transplant center. Patients were divided into 2 groups, an abnormal renal function (ARF) group (creatinine ≥1.5ng/dl; n=37) and a normal renal function (NRF) group (creatinine <1.5 ng/dl; n=31), based on creatinine of 1.5 ng/dl(132 umol/L), which was the expanded criteria donor (ECD) criterion. All organs were from deceased donors or living donors, and none of the organs were from people who were prisoners at the time of organ procurement.
This study was approved by the Ethics Committee of Tianjin First Central Hospital (No. 2017N076KY).
DATA COLLECTION:
The data collected included demographic characteristics, preoperative data, immunosuppressive regimens, early complications, clinical manifestations of PCP (fever, cough, expectoration, and dyspnea), laboratory test (serum creatinine and (1,3)-β-D-glucan antigen), imaging results, pathogen results, PCP treatment plans, ventilator support history, and clinical outcomes.
IMMUNOSUPPRESSANT AND REJECTION:
All kidney transplant patients were treated with standard immunosuppressive regimens. The induction regimen consisted of a monoclonal or polyclonal antibody alone, or combined with methylprednisolone. The maintenance immunosuppressant was cyclosporine or tacrolimus/MPA or MMF/steroid. All graft rejections were confirmed by biopsy.
CHEMOPROPHYLAXIS AND TREATMENTS:
The PCP prophylaxis regimen was daily oral administration of 80 mg TMP/400 mg SMX for 6 months starting 1 month after kidney transplantation. The standard regimen for treatment of PCP was 15–20 mg/kg/d TMP plus 75–100 mg/kg/d SMX, administered every 6–8 h. Patients with an arterial oxygen partial pressure < 70 mmHg received their treatments combined with methylprednisolone, and patients who were sensitive to TMP-SMX and had related adverse effects received a lower dose of TMP -SMX combined with caspofungin.
COMPARISON OF PROGNOSES BETWEEN THE 2 GROUPS:
The survival rates of recipients and grafts were compared between the 2 groups. The creatinine change rate was used to describe the prognosis in renal function between pre-treatment and post-treatment. The creatinine change rate was calculated by dividing the number of creatinine change (Cpost: Creatinine in post-treatment minus Cpre: Creatinine in pre-treatment) by Creatinine in pre-treatment: Change rate =(Cpost–Cpre)/Cpre*100%
STATISTICAL ANALYSIS:
Data for normally distributed continuous variables were compared using the
Results
GENERAL CHARACTERISTICS OF PATIENTS:
Between March 2011 and October 2017, 68 PCP cases (55 males and 13 females; mean age, 43±11.9 years) who met the study criteria were enrolled; 36 cases were diagnosed by microbial etiology, and 32 cases who could not tolerate bronchoscopy due to severe hypoxemia were diagnosed by clinical signs and symptoms. The median onset time of PCP was 5.6 months (range, 3.2 to 9 months) (Figure 1). Seventeen of the patients received a standard PCP prophylaxis regimen, while 51 patients did not receive a standard prophylaxis regimen due to hypoleukocytosis or a drug allergy. Eleven cases of acute rejection confirmed by biopsy were treated with methylprednisolone and 21 cases of PCP were accompanied by serum CMV positivity.
COMPARISON OF PATIENT CHARACTERISTICS BETWEEN THE 2 GROUPS:
To rule out kidney damage caused by TMP-SMX treatment, the median creatinine at 1 month prior to PCP onset was used as a criterion for group assignment, so as to better reflect pre-onset baseline renal function. The 68 patients were assigned to either the ARF group (n=37) or the NRF group (n=31) based on creatinine of 132 umol/L, which was the expanded criteria donor (ECD) criterion. The 2 groups were similar in terms of sex, BMI, donor type, induction of immunosuppression, maintenance immunosuppressant concentration, delayed graft function, and PCP symptoms (Tables 1–4). In addition, there was no significant difference between the 2 groups in proportion diagnosed by microbial etiological (20/37 [54.1%] vs. 16/31 [51.6%], p=0.841). All acute rejection that occurred before PCP was confirmed by pathology, and the rejections mostly occurred in the ARF group (10 [27%] vs. 1 [3.2%], p=0.008). TMP-SMX chemoprophylaxis was administered to 15 patients (40.5%) in the ARF group and 2 patients in the NRF group (p=0.047). Moreover, PCP patients in ARF group had longer hospital stays (25 days [17–35] vs. 20 days [14–25], p=0.03). The total lymphocyte counts decreased in both groups, but more dramatically in the ARF group (0.6 [05–0.9] vs. 1.1 [0.7–1.6], p<0.01) (Figure 2).
PROGNOSIS:
Sixty Sixty-four of the 68 patients with PCP were cured, and 4 died directly due to PCP infection. All of the deaths were in the ARF group (4/37, 10.8%). All 5 patients with graft failure were in the ARF group (5/37, 13.5%), with no significant difference between the 2 groups (Figure 3).
In this study, the rate of creatinine change before and after treatment was used to reflect the changes in renal function. There were no significant changes among the 68 cases (135.5 [110–170.5] vs. 125 [104.8–164.5], p=0.132) (Figure 4). However, the creatinine levels after treatment in each group were lower than before treatment, and the change rate was higher in the ARF group (−13.2% [−22~4.8%] vs. −4.4% [−12.6~20.9%], p=0.043) (Figure 5). Patients with a >10% decline in creatinine were mainly in the ARF group (n=23) (Figure 6).
Discussion
The acute rejection rate in the ARF group was 27% and only 3.2% in the NRF group. Wang et al. [14] found that acute rejection was a risk factor for PCP in non-lung transplant recipients, suggesting that the TMP-SMX prophylaxis regimen should be re-applied after acute rejection. Kim et al. [15] suggested that it may be beneficial to maintain 12 months of PCP prophylaxis for desensitization or acute rejection therapy in KT patients treated with rituximab. Numerous studies have shown that acute rejection is an important risk factor for PCP [16–19]. Low eGFR after renal transplantation is also considered as an important risk factor for PCP, and may be attributable to aggravation of the immunosuppressive state due to a reduction in clearance of immunosuppressive agents caused by renal dysfunction [20], as shown by the higher acute rejection rate in the ARF group. Kidney transplant recipients have more obvious renal insufficiency after rejection and receive enhanced immunosuppressive therapy, which further increases their susceptibility to PCP [16–18]. The European transplant guidelines and the KDIGO clinical practice guidelines recommend that PCP chemoprophylaxis be re-administered for 3 to 4 months after treatment for a rejection episode [3,4].
Studies have shown that lymphocytes are of great value for host resistance to PCP [13,21–23]. Iriart et al. [13] studied PCP risk factors in 33 patients who underwent solid organ transplantation (including 23 renal transplants) and identified a blood lymphocyte level of 0.75×109/L as an independent risk factor for PCP (OR=3.9 [95% CI: 1.4 to 10.7], P=0.009). Other studies found that lymphopenia is an independent risk factor for PCP [24,25]. Geertrude et al. [25] reported that lymphocyte counts might be helpful for guiding PCP prevention strategies during the first 4 months after transplantation. In the present study, the number of lymphocytes was 0.6 (0.5–0.9)×109/L in the ARF group and 1.1 (0.7–1.6)×109/L in the NRF group. We further confirmed the predictive value of lymphocytes for PCP. Moreover, lymphopenia in the ARF group may be closely related to anti-rejection therapy and enhanced immunosuppression.
TMP-SMX is the first choice for PCP prophylaxis. The incidence of PCP after renal transplantation is 5% in patients without preventive measures, while the incidence of PCP is 2% in patients with preventive measures, and PCP mortality is as high as 29–50% [26–28]. Mitsides et al. [26] reported a 38% rate of TMP-SMX withdrawal among 290 patients who underwent a standard PCP prophylaxis after renal transplantation. The main reason for discontinuation was elevated creatinine, and a 35% reversible increase in creatinine during PCP prophylaxis has been observed. Some transplantation centers do not provide routine PCP prophylaxis, mainly for the following 3 reasons: first, TMP-SMX nephrotoxicity, drug allergy, hypoleukocytosis, renal dysfunction and severe gastrointestinal adverse effects [5–7]; second, the incidence of lower PCP; and third, PCP can still occur after chemoprophylaxis [16]. In fact, there are fewer adverse effects caused by a prophylaxis dose of TMP-SMX compared to a therapeutic dose. Hyperkalemia and increased creatinine are thought to result from the inhibitory effect of trimethoprim on tubular potassium and creatinine secretion, and do not reflect true renal function [2]. In the present study, TMP-SMX standard prophylaxis was administered to 40.5% of patients in the ARF group and 6.5% in the NRF group. Postoperative renal dysfunction does not preclude the use of a standard prophylaxis regimen, but does require a more aggressive prevention regimen due to the risk for acute rejection.
In this study, 5 out of 68 patients experienced graft failure, and 4 patients died. All occurred in the ARF group, and the mortality rate was 5.8%, which is consistent with other reports [13,28]. Patients with graft failure had consistently high creatinine before PCP treatment. To reduce the renal toxicity of TMP-SMX, a small dose of TMP-SMX was administered in combination with caspofungin. A comparison of renal function between pre-treatment and post-treatment showed that 68 patients had no significant changes in creatinine, but the renal function improved after treatment. Creatinine levels after treatment decreased by 13.2% in the ARF group and by 4.4% in the NRF group, and patients with a >10% decrease were mainly in the ARF group (Figure 6). Although creatinine may rise during the early stages of TMP-SMX administration, studies have suggested that these elevations are reversible, and renal function can be slowly restored to its original state [9]. In the present study, the improvement in renal function after PCP treatment was considered to be associated with an alleviated inflammatory state and improved systemic status of the patient when infection was controlled, and was also related to the use of high-dose methylprednisolone. Although patients with renal dysfunction after renal transplantation can easily deteriorate to renal failure and death, the present study, conducted with long-term surviving patients, demonstrated that TMP-SMX nephrotoxicity does not result in a deterioration of renal function during PCP treatment. Some data support the recommendation of prolonging the prophylaxis period after transplantation [29].
The limitations of this study are its retrospective design and limited number of patients. Therefore, further prospective studies are needed to investigate individualized PCP prophylaxis regimens. However, we found that TMP-SMX prophylaxis and treatment regimens can be used in patients with renal dysfunction without significant adverse effects on long-term renal function.
Conclusions
In summary, our data indicate that PCP prophylaxis regimens in recipients after renal transplantation are still needed, regardless of whether renal function is normal or abnormal, especially for recipients with persistent lymphopenia or rejection after transplantation.
Figures
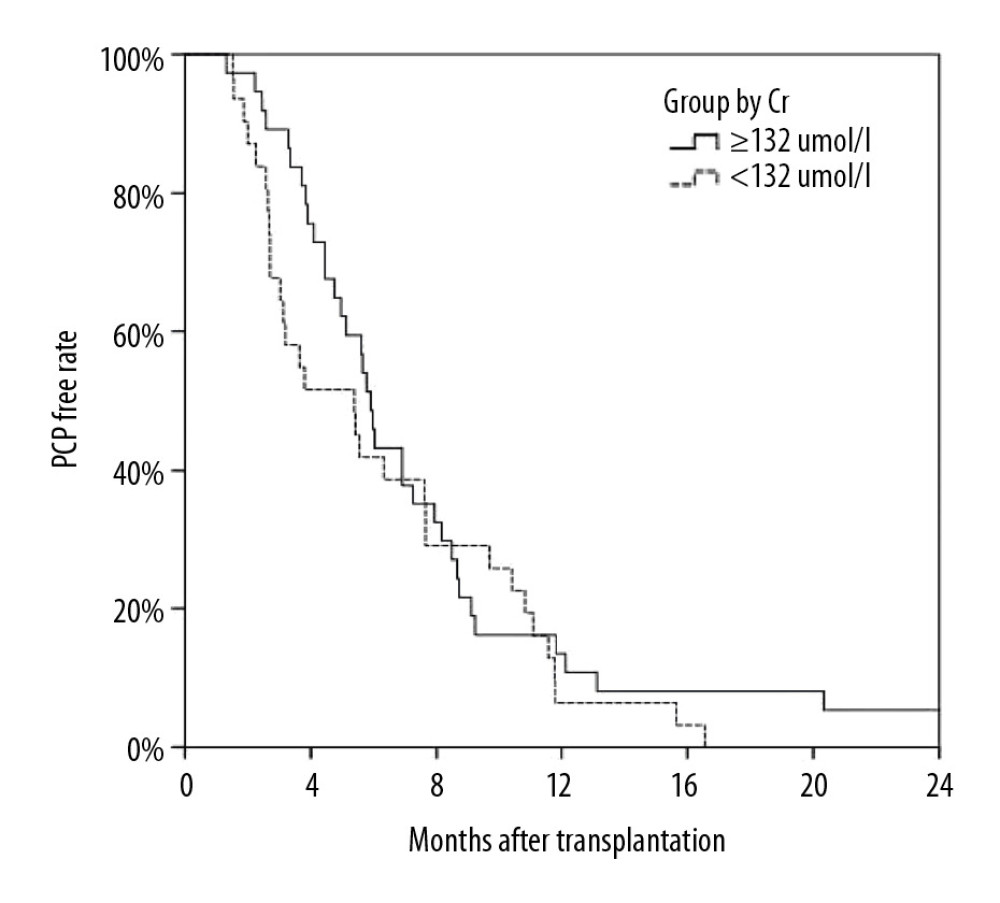 Figure 1. Kaplan-Meier curve depicting the increase in Pneumocystis pneumonia (PCP) among recipients over time after transplantation. The proportion of recipients without PCP after transplantation varied with time (months), and most cases of PCP occurred within 12 months after transplantation.
Figure 1. Kaplan-Meier curve depicting the increase in Pneumocystis pneumonia (PCP) among recipients over time after transplantation. The proportion of recipients without PCP after transplantation varied with time (months), and most cases of PCP occurred within 12 months after transplantation. 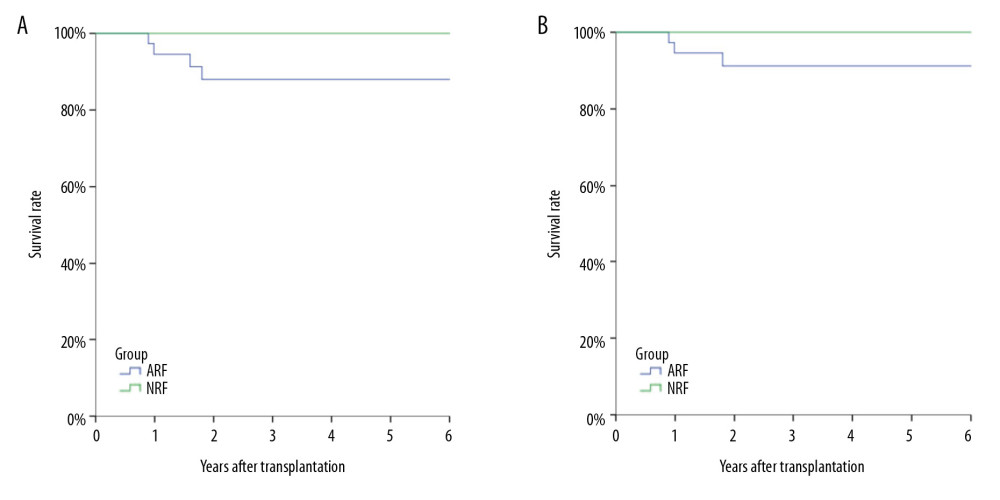 Figure 2. Analysis of long-term survival rates of grafts (A) and recipients (B) in the 2 groups. Five out of 68 patients with PCP experienced graft failure and 4 patients died, all in the ARF group.
Figure 2. Analysis of long-term survival rates of grafts (A) and recipients (B) in the 2 groups. Five out of 68 patients with PCP experienced graft failure and 4 patients died, all in the ARF group. 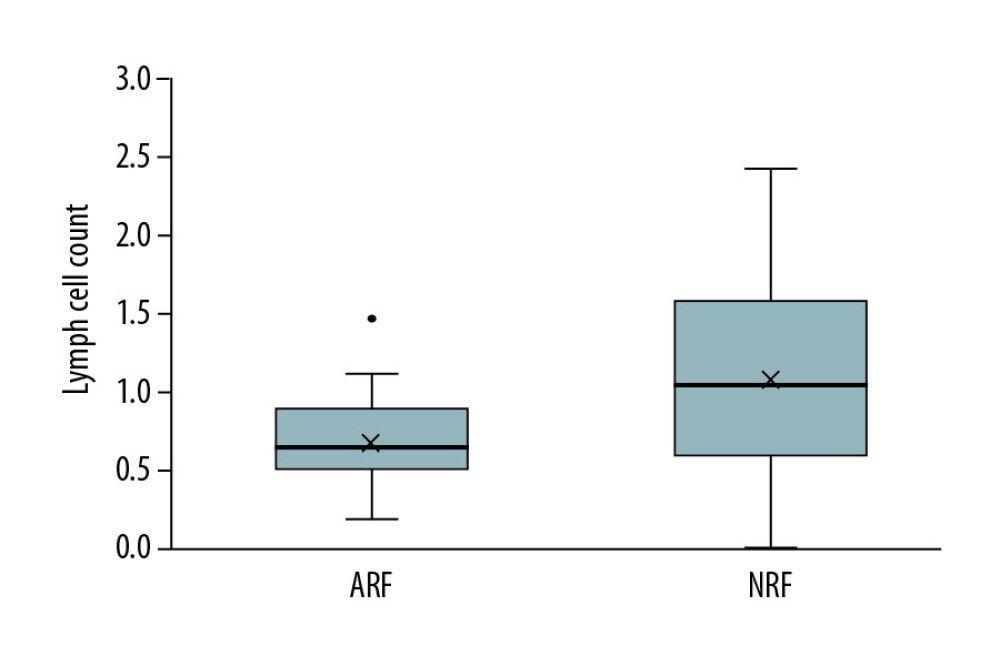 Figure 3. Comparison of lymphocyte counts in the 2 groups. Lower lymphocyte counts occurred in ARF group.
Figure 3. Comparison of lymphocyte counts in the 2 groups. Lower lymphocyte counts occurred in ARF group. 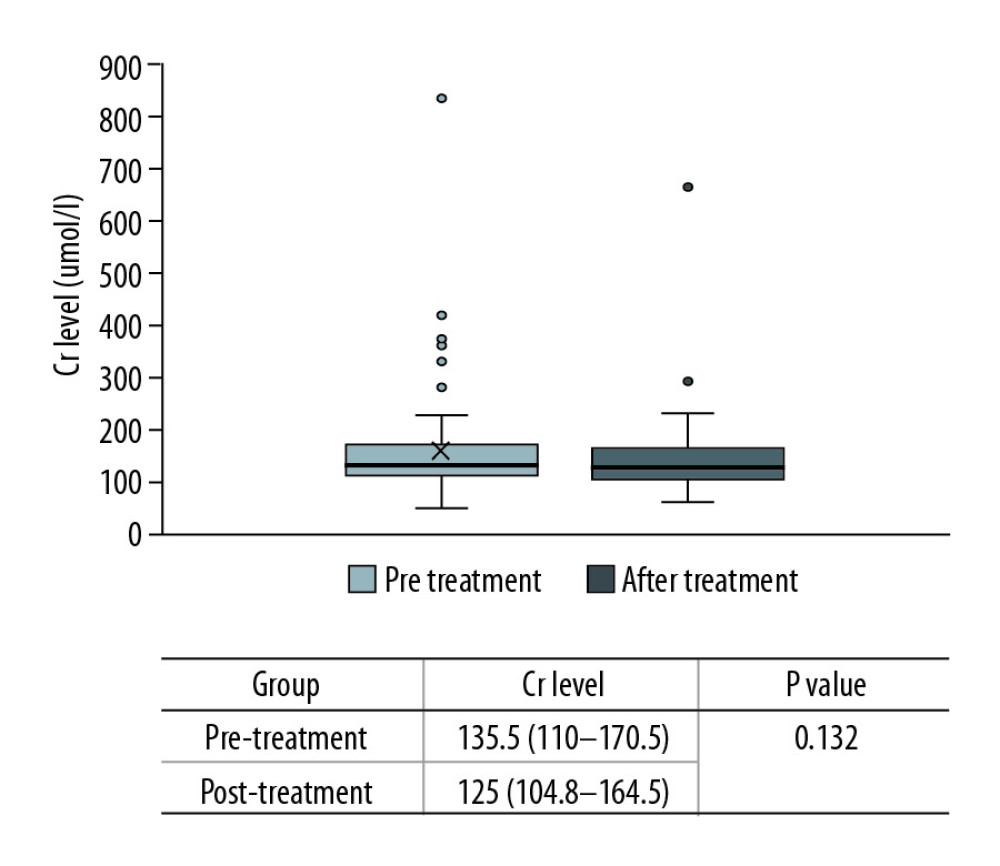 Figure 4. Comparison of renal function changes in 68 patients before and after PCP treatment as described by the creatinine change rate.
Figure 4. Comparison of renal function changes in 68 patients before and after PCP treatment as described by the creatinine change rate. 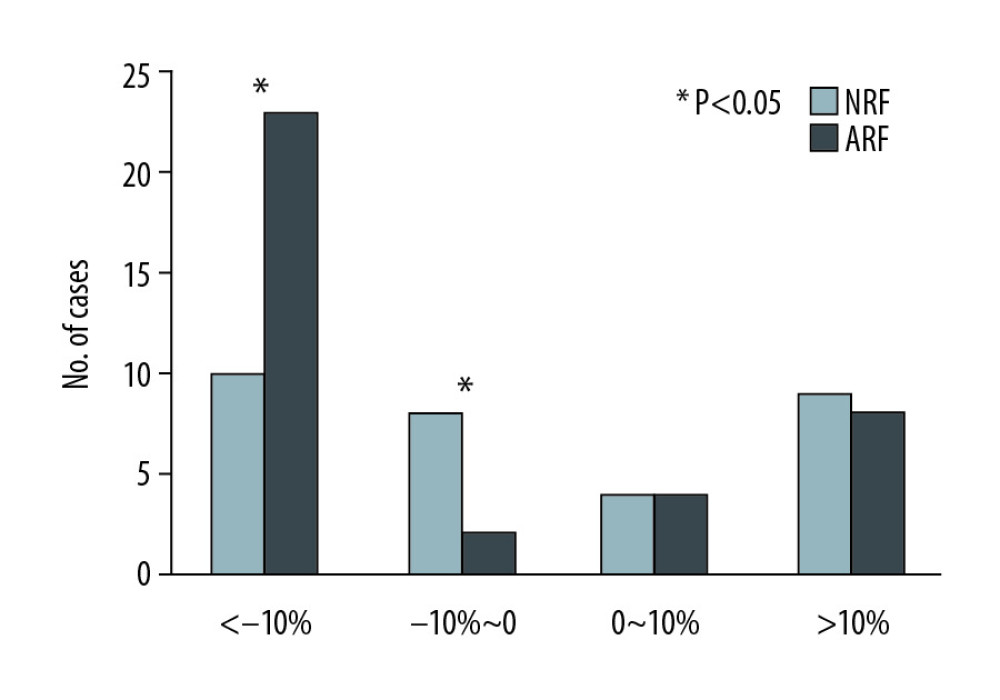 Figure 6. Comparison of creatinine change rates before and after PCP treatment. Patients with a >10% decline in creatinine were mainly in the ARF group.
Figure 6. Comparison of creatinine change rates before and after PCP treatment. Patients with a >10% decline in creatinine were mainly in the ARF group. ![Change in rate of creatinine in each group before and after PCP treatment. Creatinine was significantly lower in the ARF group (−13.2% [−22~4.8%] vs. −4.4% [−12.6~20.9%], P=0.043).](https://jours.isi-science.com/imageXml.php?i=anntransplant-25-e925126-g005.jpg&idArt=925126&w=1000) Figure 5. Change in rate of creatinine in each group before and after PCP treatment. Creatinine was significantly lower in the ARF group (−13.2% [−22~4.8%] vs. −4.4% [−12.6~20.9%], P=0.043).
Figure 5. Change in rate of creatinine in each group before and after PCP treatment. Creatinine was significantly lower in the ARF group (−13.2% [−22~4.8%] vs. −4.4% [−12.6~20.9%], P=0.043). Tables
Table 1. Comparison of general clinical data among ARF group and NRF group.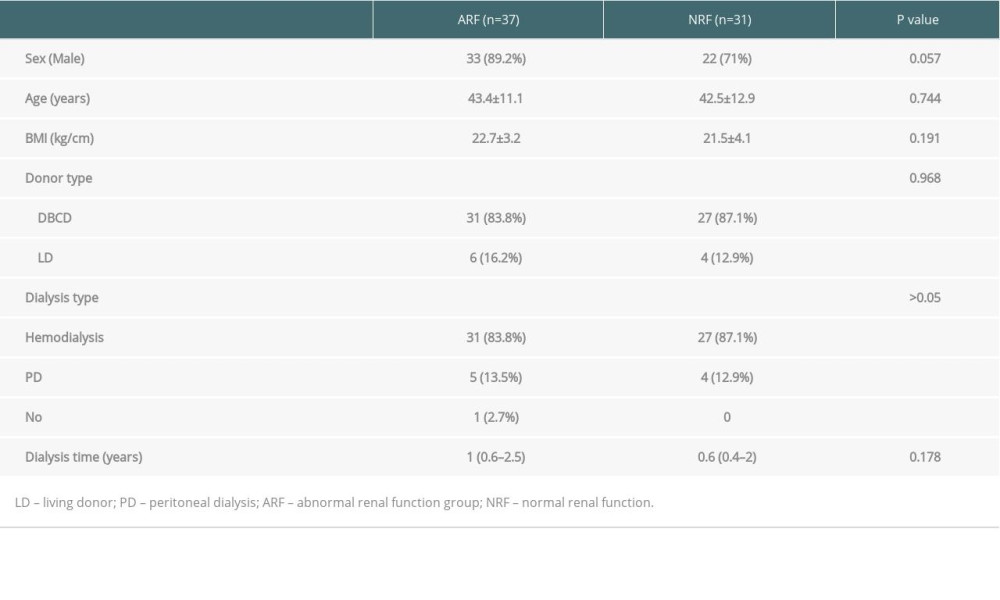 Table 2. Comparison of immunosuppressive agents and early postoperative complications among 2 groups.
Table 2. Comparison of immunosuppressive agents and early postoperative complications among 2 groups.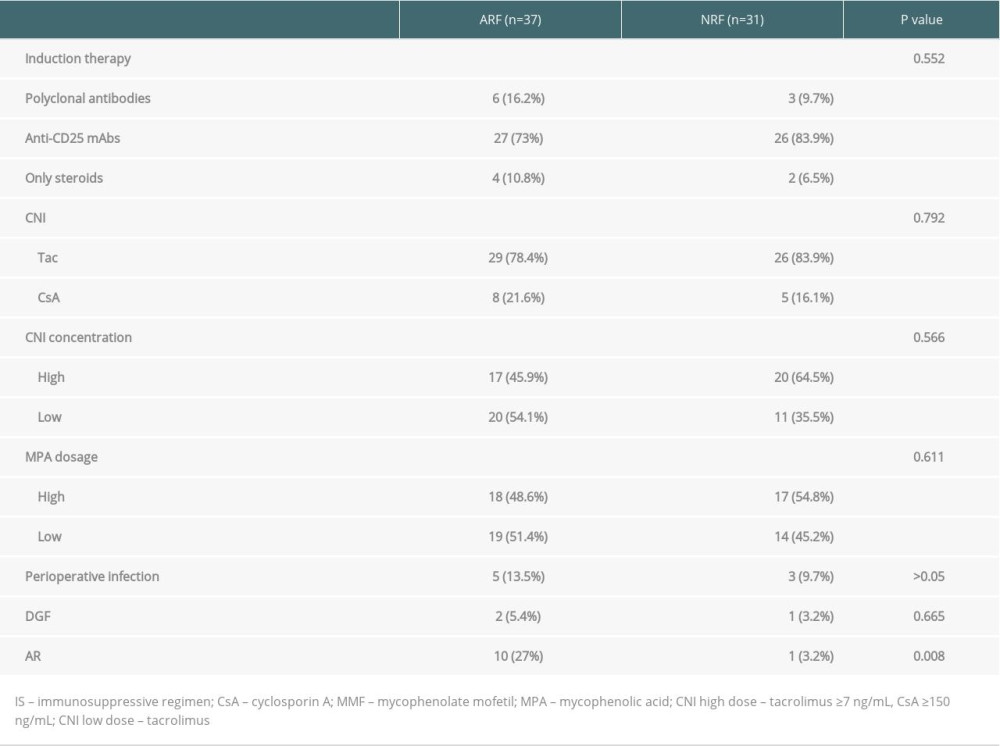 Table 3. Comparison of the onset and treatment characteristics of PCP among 2 groups.
Table 3. Comparison of the onset and treatment characteristics of PCP among 2 groups.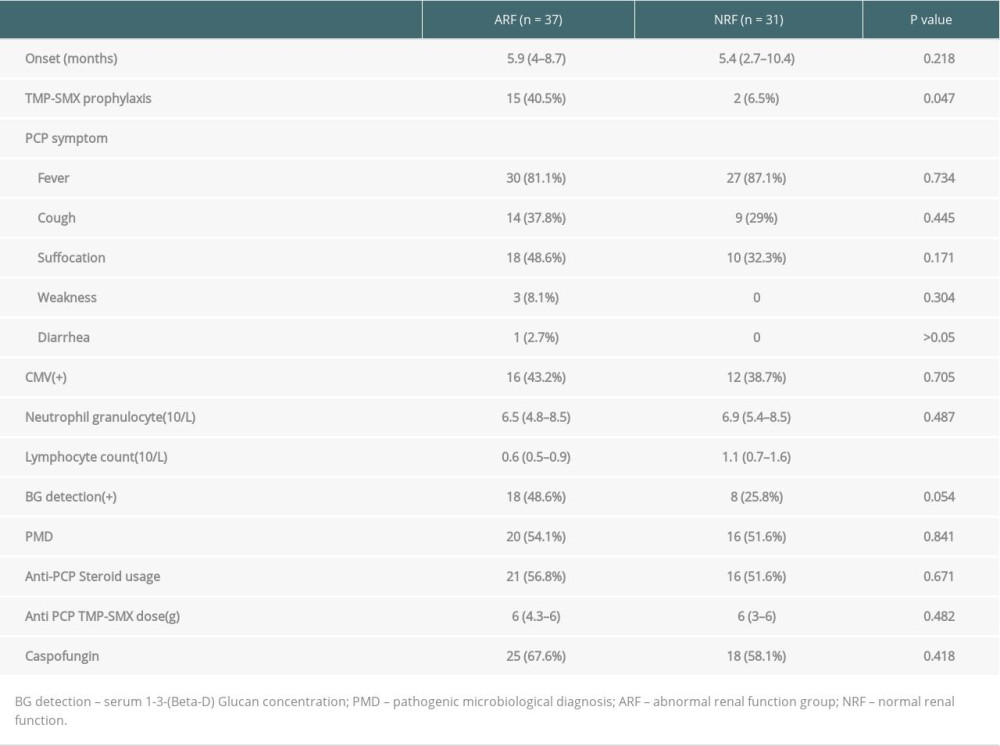 Table 4. Comparison of patient prognosis the 2 groups.
Table 4. Comparison of patient prognosis the 2 groups.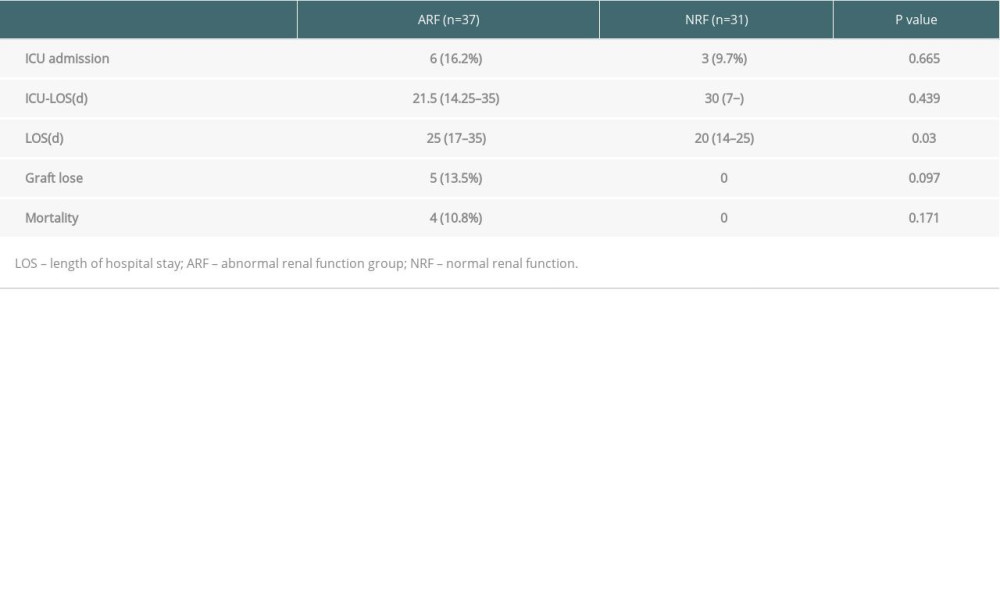
References
1. Fishman JA, Infection in solid-organ transplant recipients: N Engl J Med, 2007; 357(25); 2601-14
2. Martin SI, Fishman JA, Pneumocystis pneumonia in solid organ transplantation: Am J Transplant, 2013; 13(Suppl 4); 272-79
3. Martin SI, Fishman JA, European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.3.4. Long-term immunosuppression. Non-compliance: Nephrol Dial Transplant, 2002; 17(Suppl 4); 23-24
4. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group, KDIGO clinical practice guideline for the care of kidney transplant recipients: Am J Transplant, 2009; 9(Suppl 3); S1-155
5. Govender I, Maphasha OM, Rangiah S, Steyn C, An overview of pneumonia for the African generalist practitioner: Afr Health Sci, 2019; 19(4); 3200-7
6. Alappan R, Perazella MA, Buller GK, Hyperkalemia in hospitalized patients treated with trimethoprim-sulfamethoxazole: Ann Intern Med, 1996; 124(3); 316-20
7. Mori H, Kuroda Y, Imamura S, Hyponatremia and/or hyperkalemia in patients treated with the standard dose of trimethoprim-sulfamethoxazole: Intern Med, 2003; 42(8); 665-69
8. Antoniou T, Gomes T, Mamdami MM, Trimethoprim-sulfamethoxazole induced hyperkalemia in elderly patients receiving spironolactone: Nested case-control study: BMJ, 2011; 343; d5228
9. Gordon SM, LaRosa SP, Kalmadi S: Clin Infect Dis, 1999; 28(2); 240-46
10. Van Gundy K, Boylen CT, Fiberoptic bronchoscopy. Indications, complications, contraindications: Postgrad Med, 1988; 83(1); 289-94
11. Fulkerson WJ, Current concepts. Fiberoptic bronchoscopy: N Engl J Med, 1984; 311(8); 511-15
12. Carmona EM, Limper AH: Ther Adv Respirat Dis, 2011; 5(1); 41-59
13. Iriart X, Challan Belva T, Fillaux J: Am J Transplant, 2015; 15(1); 190-99
14. Wang EHZ, Partovi N, Levy RD, Pneumocystis pneumonia in solid organ transplant recipients: not yet an infection of the past: Transpl Infect Dis, 2012; 14(5); 519-25
15. Kim YH, Kim JY, Kim DH: BMC Nephrol, 2020; 21(1); 93
16. Eitner F, Hauser IA, Rettkowski O, Rath T: Nephrol Dial Transplant, 2011; 26(6); 2013-17
17. Radisic M, Lattes R, Chapman JF: Transpl Infect Dis, 2003; 5(2); 84-93
18. de Boer MGJ, Kroon FP, Cessie SI: Transpl Infect Dis, 2011; 13(6); 559-69
19. Arend SM, Westendorp RGJ, Kroon FP: Clin Infect Dis, 1996; 22(6); 920-25
20. Kuypers DRJ, Mourad M, Vanrenterghem Y, Twelve-month evaluation of the clinical pharmacokinetics of total and free mycophenolic acid and its glucuronide metabolites in renal allograft recipients on low dose tacrolimus in combination with mycophenolate mofetil: Ther Drug Monit, 2003; 25(5); 609-22
21. Freiwald T, Büttner S, Büttner S, CD4 T cell lymphopenia predicts mortality from Pneumocystis pneumonia in kidney transplant patients: Clin Transplant, 2020 [Online ahead of print]
22. Evernden C, Dowhan M, Dabas R: Cytotherapy, 2020; 22(1); 27-34
23. Limper AH: Am J Respir Crit Care Med, 2018 [Online ahead of print]
24. Hertoghs KML, Moerland PD, van Stijn A, Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation: J Clin Invest, 2010; 120(11); 4077-90
25. Struijk GH, Gijsen AF, Yong SL: Nephrol Dial Transplant, 2011; 26(10); 3391-98
26. Mitsides N, Greenan K, Green D, Complications and outcomes of trimethoprim-sulphamethoxazole as chemoprophylaxis for pneumocystis pneumonia in renal transplant recipients: Nephrology (Carlton, Vic), 2014; 19(3); 157-63
27. Kovacs JA, Masur H, Evolving health effects of Pneumocystis: One hundred years of progress in diagnosis and treatment: JAMA, 2009; 301(24); 2578-85
28. Thomas CF, Limper AH, Pneumocystis pneumonia: N Engl J Med, 2004; 350(24); 2487-98
29. Chapman FA, Dickerson JE, Daly C, Impact of increased duration of trimethoprim-sulfamethoxazole prophylaxis for pneumocystis pneumonia after renal transplant: Ann Transplant, 2019; 24; 625-30
Figures
 Figure 1. Kaplan-Meier curve depicting the increase in Pneumocystis pneumonia (PCP) among recipients over time after transplantation. The proportion of recipients without PCP after transplantation varied with time (months), and most cases of PCP occurred within 12 months after transplantation.
Figure 1. Kaplan-Meier curve depicting the increase in Pneumocystis pneumonia (PCP) among recipients over time after transplantation. The proportion of recipients without PCP after transplantation varied with time (months), and most cases of PCP occurred within 12 months after transplantation. Figure 2. Analysis of long-term survival rates of grafts (A) and recipients (B) in the 2 groups. Five out of 68 patients with PCP experienced graft failure and 4 patients died, all in the ARF group.
Figure 2. Analysis of long-term survival rates of grafts (A) and recipients (B) in the 2 groups. Five out of 68 patients with PCP experienced graft failure and 4 patients died, all in the ARF group. Figure 3. Comparison of lymphocyte counts in the 2 groups. Lower lymphocyte counts occurred in ARF group.
Figure 3. Comparison of lymphocyte counts in the 2 groups. Lower lymphocyte counts occurred in ARF group. Figure 4. Comparison of renal function changes in 68 patients before and after PCP treatment as described by the creatinine change rate.
Figure 4. Comparison of renal function changes in 68 patients before and after PCP treatment as described by the creatinine change rate. Figure 6. Comparison of creatinine change rates before and after PCP treatment. Patients with a >10% decline in creatinine were mainly in the ARF group.
Figure 6. Comparison of creatinine change rates before and after PCP treatment. Patients with a >10% decline in creatinine were mainly in the ARF group.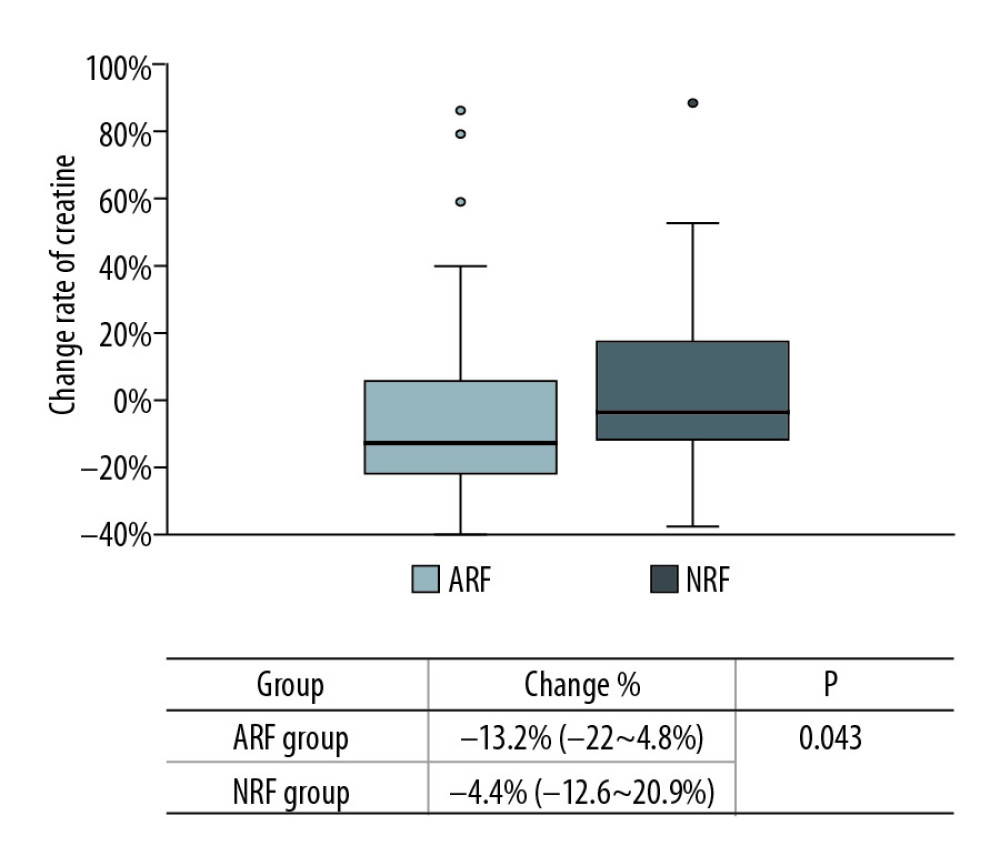 Figure 5. Change in rate of creatinine in each group before and after PCP treatment. Creatinine was significantly lower in the ARF group (−13.2% [−22~4.8%] vs. −4.4% [−12.6~20.9%], P=0.043).
Figure 5. Change in rate of creatinine in each group before and after PCP treatment. Creatinine was significantly lower in the ARF group (−13.2% [−22~4.8%] vs. −4.4% [−12.6~20.9%], P=0.043). Tables
 Table 1. Comparison of general clinical data among ARF group and NRF group.
Table 1. Comparison of general clinical data among ARF group and NRF group. Table 2. Comparison of immunosuppressive agents and early postoperative complications among 2 groups.
Table 2. Comparison of immunosuppressive agents and early postoperative complications among 2 groups. Table 3. Comparison of the onset and treatment characteristics of PCP among 2 groups.
Table 3. Comparison of the onset and treatment characteristics of PCP among 2 groups. Table 4. Comparison of patient prognosis the 2 groups.
Table 4. Comparison of patient prognosis the 2 groups. Table 1. Comparison of general clinical data among ARF group and NRF group.
Table 1. Comparison of general clinical data among ARF group and NRF group. Table 2. Comparison of immunosuppressive agents and early postoperative complications among 2 groups.
Table 2. Comparison of immunosuppressive agents and early postoperative complications among 2 groups. Table 3. Comparison of the onset and treatment characteristics of PCP among 2 groups.
Table 3. Comparison of the onset and treatment characteristics of PCP among 2 groups. Table 4. Comparison of patient prognosis the 2 groups.
Table 4. Comparison of patient prognosis the 2 groups. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








