09 March 2021: Original Paper
Urinary C-X-C Motif Chemokine 10 Is Related to Acute Graft Lesions Secondary to T Cell- and Antibody-Mediated Damage
Alvaro Arnau1BEF*, Adalberto Benito-Hernández1ABCDEFG, María Angeles Ramos-Barrón1BCDF, María Teresa García-Unzueta2BCDF, José Javier Gómez-Román3BCDF, José María Gómez-Ortega3BCDF, Marcos López-Hoyos4BCDFG, David San Segundo4BCDF, Juan Carlos Ruiz1BCDFG, Emilio Rodrigo1ABCDEFGDOI: 10.12659/AOT.929491
Ann Transplant 2021; 26:e929491
Abstract
BACKGROUND: Non-invasive biomarkers of graft rejection are needed to optimize the management and outcomes of kidney transplant recipients. Urinary excretion of IFN-g-related chemokine CXCL10 is clearly associated with clinical and subclinical T cell-mediated graft inflammation, but its relationship with antibody-mediated damage has not been fully addressed. Further, the variables influencing levels of urinary CXCL10 excretion are unknown.
MATERIAL AND METHODS: A total of 151 kidney graft biopsies (92 surveillance and 59 indication biopsies) and 151 matched urine samples obtained before biopsy were prospectively analyzed. T cell-mediated rejection (TCMR) and antibody-mediated rejection (AbMR) were defined according to the 2017 Banff classification criteria. Urinary CXCL10 levels were measured by ELISA and corrected by urinary creatinine.
RESULTS: Banff scores ‘t’, ‘i’, ‘g’, and ‘ptc’ were significantly related to urinary CXCL10 levels. Multivariate analysis showed that ‘t’ (β=0.107, P=0.001) and ‘ptc’ (β=0.093, P=0.002) were significantly associated with urinary CXCL10. Donor-specific antibodies (DSAs) were related to the high excretion of urinary CXCL10 at 1 year after transplantation (odds ratio [OR] 17.817, P=0.003). Urinary CXCL10 showed good discrimination ability for AbMR (AUC-ROC 0.760, P=0.001). The third tertile of urinary CXCL10 remained significantly associated with AbMR (OR 4.577, 95% confidence interval 1.799–11.646, P=0.001) after multivariate regression analysis.
CONCLUSIONS: DSA was the only variable clearly related to high urinary CXCL10 levels. Urinary CXCL10 is a good non-invasive candidate biomarker of AbMR and TCMR, supplying information independent of renal function and other variables normally used to monitor kidney transplants.
Keywords: Biological Markers, Chemokine CXCL10, Graft Rejection, Kidney Transplantation, Antibodies, T-Lymphocytes
Background
Kidney transplantation is the best therapy for patients with end-stage renal disease. The need for patients to return to dialysis after kidney graft failure is a significant problem and is associated with a high mortality rate, sensitization, deterioration of quality of life, and necessity of relisting on the transplant waiting list [1,2]. Although the kidney graft half-life has improved in recent years, this has been mainly due to better short-term graft survival, whereas the occurrence rate of long-term graft loss has remained stable [3]. Alloimmune-mediated damage has been identified as the main mechanism responsible for most graft losses [4,5]. A recent study reported that unspecific chronic injury is found in up to 21% of the last biopsies performed before graft loss and identified that a previous rejection episode had occurred in 73% of these patients [6].
The routine follow-up of kidney transplant recipients has traditionally consisted of monitoring serum creatinine, immunosuppressive blood levels, urinary sediment, and protein excretion, and, more recently, BK poliomavirus viremia and donor-specific antibodies (DSAs) [7,8]. However, it is known that some kidney transplant recipients can develop significant cellular-mediated or antibody-mediated inflammation, which is detectable only by an invasive test such as a kidney biopsy [9]. This subclinical inflammation can lead to further graft damage and graft loss [10–12], and its therapy can improve the outcome of the kidney graft [12–14]. Unfortunately, surveillance biopsies (also known as protocol or for-cause biopsies) of the kidney graft create risks to the patient and are expensive and cumbersome, limiting the possibility of repeating the procedure [15]. Moreover, sampling error and a lack of agreement among pathologists can reduce the usefulness of surveillance biopsies [16]. Therefore, non-invasive and objective biomarkers are needed in the field of kidney transplantation for the precise diagnosis and monitoring of patients [17]. There are some serum biomarkers that relate to different histological findings, but urine biomarkers could be preferable for following kidney transplant recipients because urine sampling is a truly non-invasive technique, allowing for repeatability. Also, urine molecules reflect the activity taking place inside the kidney graft. Among the urinary biomarkers, the IFN-γ-related chemokines C-X-C motif chemokine 9 (CXCL9) and CXCL10 have received much attention and have been analyzed at the RNA and protein level. Many studies found a clear relationship between these chemokines and clinical and subclinical T cell-mediated graft inflammation [18–37]. Since 2015, 3 studies have reported that CXCCL10 relates not only to T cell-mediated inflammation but also to antibody-mediated graft damage [30,31,35], although the results of 2 other studies did not find the same relationship [26,27]. However, these 5 studies did not use the latest 2017 Banff classification of antibody-mediated rejection (AbMR) [38]. Also, the factors related to the levels of CXCL10 were not fully addressed in these studies or elsewhere.
We performed a prospective study to analyze the relationship between AbMR, classified according to the 2017 Banff criteria of AbMR, and the urinary excretion levels of CXCL10 and the histological findings of indication and surveillance biopsies. We also analyzed the potential factors related to urinary CXCL10 values, focusing on immunosuppressive drug-related variables.
Material and Methods
STATISTICAL ANALYSIS:
Continuous variables were expressed as mean±standard deviation. Categorical variables were described as relative frequencies. CXCL10/Cr was analyzed as a continuous and a dichotomic variable (the highest tertile vs medium and lowest tertiles). The Spearman’s rank correlation coefficient and an unsupervised hierarchical clustering analysis were used to explore the relationship between urinary CXCL10/Cr and Banff scores. Multivariate lineal regression analysis was used to find the relationship of individual Banff scores with the logarithm of urinary CXCL10/Cr. Variables related with the highest tertile of urinary CXCL10/Cr were analyzed by
Results
URINARY CXCL10/CR RELATES TO TUBULAR AND PERITUBULAR INFLAMMATION:
The results of the Spearman correlation analysis between the logarithm of urinary CXCL10/Cr and Banff scores are shown in Table 2. Most acute Banff scores were significantly correlated with urinary CXCL10/Cr. The sum of scores of the tubular and peritubular capillary compartment (‘t’+‘ptc’) was also significantly correlated with urinary CXCL10/Cr (rho=0.360, P=0.001). Acute Banff score (‘t’+‘i’+‘v’+‘g’) was correlated with urinary CXCL10/Cr (rho=0.390, P<0.001), whereas chronic Banff score (‘ct’+‘ci’+‘cv’+‘cg’) was not correlated with urinary CXCL10/Cr (rho=0.104, P=0.209). Multivariate lineal regression analysis results showed that both ‘t’ (β=0.107, 95% confidence interval [CI] 0.042–0.171, P=0.001) and ‘ptc’ (β=0.093, 95% CI 0.034–0.152, P=0.002) Banff scores were significantly associated with the logarithm of urinary CXCL10/Cr. Unsupervised hierarchical clustering analysis (Figure 1) of Banff scores and urinary CXCL10/Cr levels showed that CXCL10/Cr was highly associated with Banff scores related to both T cell-mediated inflammation (‘t’, ‘i’) and antibody-mediated damage (‘ptc’, ‘g’, and ‘cg’).
RISK FACTORS RELATED TO 1-YEAR URINARY CXCL10/CR IN SURVEILLANCE BIOPSIES:
The analysis of variables associated with the highest tertile of CXCL10/Cr is shown in Table 3. In a multivariate logistic regression model including all variables with a P value under 0.2, only cold ischemia time (odds ratio [OR] 1.101, 95% CI 1.012–1.197, P=0.025), urinary leukocyte count (OR 1.037, 95% CI 1.004–1.072, P=0.028), and DSA at biopsy (OR 17.817, 95% CI 2.593–122.415, P=0.003) were related to the highest tertile of urinary CXCL10/Cr at 1 year. Whereas the female sex showed significantly higher values of urinary CXCL10/Cr (17.93±20.85 vs 10.66±8.21 P=0.009) by the Mann-Whitney U test, this relationship disappeared after adjusting by the number of urinary leucocytes at 1 year. Only 2 patients showed BK nephropathy, and their CXCL10/Cr values were not significantly increased.
URINARY CXCL10/CR IDENTIFIED CLINICAL AND SUBCLINICAL ABMR INDEPENDENTLY OF OTHER VARIABLES:
The variables related to the subclinical and clinical histologic findings of AbMR are shown in Table 1. The diagnostic performance of CXCL10/Cr in predicting clinical and subclinical AbMR was estimated by ROC curve analysis. The area under the curve (AUC)-ROC value was 0.760 (95% CI 0.683–0.836, P<0.001) (Figure 2). The AUC-ROC value of the glomerular filtration rate (GFR) was 0.709 (95% CI 0.625–0.794, P<0.001). The value of CXCL10/Cr that showed the best sensitivity (75.0%) and specificity (70.7%) was 11.95 ng/mmoL. After adjusting by recipient age, live donation, DCD, retransplantation, renal function and proteinuria, pretransplant panel-reactive antibodies, and leukocyte count, the multivariate logistic regression analysis results showed that the highest tertile remained significantly associated with AbMR (OR 4.577, 95% CI 1.799–11.646, P=0.001), retransplantation (OR 3.886, 95% CI 1.508–10.011, P=0.005), GFR (OR 0.975, 95% CI 0.954–0.995, P=0.016), and proteinuria (P=0.014).
A similar analysis was performed for surveillance biopsies. The variables related to subclinical AbMR are shown in Table 4. The diagnostic performance of CXCL10/Cr in predicting clinical and subclinical AbMR was estimated by ROC curve analysis. The AUC-ROC value was 0.799 (95% CI 0.702–0.896, P<0.001) (Figure 3), whereas the AUC-ROC value of GFR was 0.767 (95% CI 0.656–0.878, P<0.001). The CXCL10/Cr value that showed the best sensitivity (82.9%) and specificity (78.3%) was 11.90 ng/mmoL. Multivariate logistic regression analysis demonstrated that the highest tertile remained significantly associated with AbMR (OR 9.729, 95% CI 2.134–44.351, P=0.003) and was independent of other variables, including retransplantation, renal function, and urinary leukocyte count.
URINARY CXCL10/CR IDENTIFIED CLINICAL AND SUBCLINICAL TCMR INDEPENDENTLY OF OTHER VARIABLES:
In total, 36 (23.8%) biopsies showed TCMR. The ROC curve demonstrated that CXCL10/Cr was able to discriminate biopsies with both clinical and subclinical TCMR (AUC-ROC 0.719, 95% CI 0.630–0.808, P<0.001) (Figure 4). The CXCL10/Cr value that showed the best sensitivity (72.2%) and specificity (67.8%) was 13.30 ng/mmoL. The multivariate logistic regression analysis results showed the third tertile of urinary CXCL10/Cr at biopsy was significantly related to the histologic diagnosis of clinical and subclinical TCMR (OR 2.505, 95% CI 1.048–5.992, P=0.039) and was independent of other variables, including recipient age, sex, renal function, live donation, the presence of DSA at biopsy, and the urinary leukocyte number.
Among the surveillance biopsies, 15 (16.3%) showed signs of subclinical TCMR. CXCL10/Cr was also able to discriminate between patients with and without subclinical TCMR (AUC-ROC 0.779, 95% CI 0.651–0.907,
URINARY CXCL10/CR IDENTIFIED ACUTE HISTOLOGIC ACTIVITY IN DSA-POSITIVE PATIENTS:
A total of 41 patients were positive for DSAs at biopsy. Among them, patients in the third tertile of urinary CXCL10/Cr at biopsy showed the highest scores, compared with those of patients in the lower tertiles, of ‘t’ (0.81±0.81 vs 1.40±0.68, P=0.016), ‘g’ (0.71±0.85 vs 1.35±0.88, P=0.023), and the composite acute Banff score (3.62±2.31 vs 5.20±2.24, P=0.032) (Figure 5). There were no significant differences among the other acute Banff scores or any chronic score.
SIX-MONTH URINARY CXCL10/CR PREDICTED SUBCLINICAL HISTOLOGIC FINDINGS AT 1 YEAR:
Among the patients who had surveillance biopsies, values of urinary CXCL10/Cr at 6 months after transplantation were available in 41 patients. Urinary CXCL10/Cr at 6 months was also useful to identify those patients who would have subclinical AbMR (AUC-ROC 0.773, 95% CI 0.596–0.949, P=0.008) and subclinical TCMR (AUC-ROC 0.716, 95% CI 0.537–0.895, P=0.042) (Figures 6, 7, respectively).
Discussion
Similar to the results of previous studies, we found that the urinary chemokine CXCL10 related to acute Banff injury more than to chronic Banff injury [23–26,30,31,35]. The acute individual scores associated with urinary CXCL10/Cr were ‘t’, ‘i’, ‘g’, and ‘ptc’. The only acute score in the present study that did not relate to urinary CXCL10/Cr was ‘v’, as was also reported by Hirt-Minkowski et al [26] and Ho et al [35]. Moreover, a composite acute score was correlated with urinary CXCL10/Cr, whereas the composite chronic score was not. Antibody-mediated and cellular inflammatory scores showed this relationship by Spearman’s correlation analysis. Also, cluster analysis graphically demonstrated the proximity of urinary CXCL10/Cr to cellular-mediated (‘t’ and ‘i’) and antibody-mediated (‘g’ and ‘ptc’) inflammation. This finding was also reported by Rabant et al [30]. In the present study, the only chronic score associated with urinary CXCL10 was ‘cg’. Although this score is closely related to ‘g’ and ‘ptc’, it is known that AbMR is a continuous process induced by DSAs without a clear differentiation point between the active and chronic phases of damage [40,41]. As pointed out by Rabant et al and Ho et al, urinary CXCL10 reflects the inflammatory damage of the tubular compartment (‘t’ and ‘ptc’), a finding supported by the results of our Spearman correlation analysis (rho=0.360,
Because urinary CXCL10 is a potential marker of the events taking place inside the kidney graft, we investigated the variables that relate to urinary CXCL10. Female sex, cold ischemia time, the presence of DSA at biopsy, urinary leukocyturia, and renal function were associated with urinary CXCL10, according to univariate analysis results. Although female sex was associated with a higher level of urinary CXCL10, this relationship disappeared after adjusting for urinary leukocyte number, probably because women who receive transplants have urinary tract infections more frequently than do men. The relationship between urinary leukocyte count and urinary CXCL10 excretion has been reported as being due to urinary chemokines coming not only from the kidney but also from the white blood cells found in the urine [42]. Owing to the low prevalence of BK nephropathy in our kidney transplant recipients, we did not find the association between polyomavirus infection and urinary CXCL10 that was reported in other studies [18,25,29,31,42]. The finding that patients with a longer cold ischemia time had higher values of CXCL10 at 1 year after transplantation does not have a clear explanation. Although it is known that ischemia-reperfusion can promote an alloimmune response, the delayed graft function rate was not significantly higher in patients in the higher tertile of urinary CXCL10 excretion. Like previous authors, we found in the present study that urinary CXCL10 was also higher in stable patients with subtly worse renal function in whom the renal biopsy was performed for surveillance, although the relationship between urinary CXCL10 and renal function has not been observed by all authors [31,42].
In relation with the alloimmune response, the only variable related to a higher excretion of urinary CXCL10 was the presence of DSA. This was also highlighted by Hirt-Minkowski et al, who reported that patients with DSA had significantly higher urinary CXCL10 levels compared with DSA-negative patients (median, 1.8 ng/mmoL vs 0.8 ng/mmoL,
The main finding of our study was that urinary CXCL10 was strongly related to a histological diagnosis of AbMR, confirming the results of previous [30,31,35], but not all studies [26,27]. In our study, urinary CXCL10 showed good discrimination for histological AbMR with an AUC-ROC value of 0.760 for indication and surveillance biopsies combined (and 0.799 for only surveillance biopsies). These values are similar to those reported by Rabant et al (0.755) [30] and Ho et al (0.70) [35]. Good sensitivities and specificities of different cutoff values suggest that urinary CXCL10 could be an effective non-invasive biomarker to differentiate kidney transplant recipients with antibody-mediated damage. Interestingly, the information provided for urinary CXCL10 excretion levels is independent of the other variables currently used to monitor kidney graft outcome, such as renal function, proteinuria, and immunosuppressive drug levels. Also, the relationship between urinary CXCL10 level and AbMR was not dependent on a single reported confounding factor, such as urinary leukocyte count [42]. Our present results suggest that those kidney transplant recipients in the highest tertile of urinary CXCL10 excretion have more than 4 times the risk of having AbMR in any type of biopsy and more than 9 times the risk of having AbMR in a surveillance biopsy. Conversely, a low urinary CXCL10 level is a sign of a quiescent state in which a surveillance biopsy was less likely to detect antibody-mediated allograft damage [33].
Our results also showed that urinary CXCL10 was clearly associated with a higher risk of TCMR, whereby kidney recipients in the highest tertile of urinary CXCL10 had a 2.5 times higher risk of TCMR than did patients in a lower tertile. Urinary chemokine level showed a good discrimination ability to detect TCMR, with a global AUC-ROC value of 0.719 (0.779, limiting the analysis to only surveillance biopsies). Previous studies reported AUC-ROC values ranging from 0.681 to 0.930 [19–22,24,26,28,30–34]. As with AbMR, urinary CXCL10 excretion was independently related to TCMR without other variables such as renal function and leukocyturia [18,26].
The development of post-transplant de novo DSA has been recognized as a main cause of further kidney graft failure. Human leukocyte antigen mismatches, previous rejections, immunosuppressive medication nonadherence, or lower exposure of tacrolimus blood levels are known risk factors of kidney graft failure [43–46]. Once DSAs appear, it is possible to detect AbMR in 25% of patients, although the rate will increase up to 50% after 1 year [47]. Conversely, a significant number of patients with de novo DSA will not suffer a rejection episode. In this sense, it would be interesting to know in which patients DSAs lead to allograft damage. Although we had a limited sample, we demonstrated that kidney graft recipients with circulating DSAs and with high urinary CXCL10 excretion had more tubulitis and glomerulitis than did DSA-positive patients in the lower CXCL10 tertiles. The presence of tubulitis in patients is a known risk factor for graft loss in patients with de novo DSA [48]. If this finding is confirmed in a larger study, urinary CXCL10 could be used to determine which patients would have DSA-induced injury in the allografts by a non-invasive technique.
It has been reported that urinary CXCL10 can also predict long-term graft outcome [29] and the histological findings of subsequent biopsies [29,33,36]. Urinary CXCL10 at 6 months after kidney transplantation showed a good discrimination ability for TCMR and AbMR at 1 year, with AUC-ROC values of 0.716 and 0.773, respectively. This prediction ability was similar to that reported by Rabant et al In their study, urinary CXCL10 at 3 months predicted further clinical and subclinical acute rejection episodes independently of renal function with TCMR and AbMR AUC-ROC values of 0.73 and 0.66, respectively [33]. In fact, urinary CXCL10 at 6 months performed like urinary CXCL10 at 1 year for predicting subclinical rejection. Some sequential studies reported that urinary CXCL10 started to increase from between 1 week to several months before cellular rejection [20,27,33]. The timing of elevation of urinary CXCL10 before clinical AbMR has not been previously defined. Because AbMR is a continuous process, it seems logical that more than predicting AbMR, a high urinary CXCL10 level is a marker of acute inflammatory phenomena taking place inside the kidney graft, which will be only uncovered by a surveillance biopsy.
Although logistic regression analysis and ROC curve analysis demonstrated that urinary CXCL10 was independently related to TCMR and AbMR and showed good discrimination ability for both situations, urinary CXCL10 cannot replace a kidney graft biopsy for diagnosing both entities. Even some patients in the highest tertile of urinary CXCL10 showed an absence of histological allograft damage, while some patients in lower tertiles of CXCL10 could develop TCMR or AbMR. However, urinary CXCL10 was closely related to the acute histological findings, independent of other variables. In fact, urinary CXCL10 was not only related with TCMR and AbMR independent of renal function measurement, it also showed a comparable or even slightly better discrimination ability than GFR. Also, urinary CXCL10 is a completely non-invasive biomarker. Which raises the question: why has the measurement of CXCL10 not increased in use to monitor kidney transplant recipients together with creatinine, proteinuria, immunosuppressive drug levels, BK viremia, and DSA? The main reasons for this could be that the currently available techniques to measure CXCL10 protein by ELISA or its RNA by PCR are time-consuming and more expensive than the other kidney graft biomarkers. Because urinary CXCL10 is a better biomarker of the types of inflammation inside the kidney graft than CXCL9 [30], new technological developments applied to urinary CXCL10 detection, such as the use of rapid biolayer interferometry for measuring CXCL9, can help to popularize the monitoring of urinary chemokines in the kidney transplant field [49].
Our study has some limitations. First, being a single-center study, the sample size is small, and the data cannot be generalized to different populations without confirmatory studies. However, our findings are similar to those previously reported, reinforcing the relationship between urinary CXCL10 and histological findings. Second, because the study included indication and surveillance biopsies, some patients were biopsied twice, which could have falsely bolstered the relationship between urinary CXCL10 and TCMR and AbMR. However, the findings were very similar when we analyzed only surveillance biopsies with only 1 biopsy per patient. Finally, the rate of subclinical AbMR at 1 year was as high as 25%. Banff 2017 classification incorporated C4d positivity as an alternative for DSA criterion in cases of potentially false-negative DSA [38], increasing our subclinical AbMR rejection rate. In our center, molecular AbMR assessment is not available, and some of these subclinical cases of AbMR could have been misclassified. Also, we cannot dismiss the possibility that some patients are more prone to accept a surveillance biopsy when they have some previous risks or when they are experiencing some subtle deterioration of renal function. Being a relatively small center, we find that our kidney transplant population is characterized by a high rate of retransplants, with previous sensitization before transplantation. Also, we found a higher rate of AbMR (49%) than of TCMR (36%) in the indication biopsies. This was because less than 30% of the indication biopsies were made the first 6 months after transplantation. In fact, mean time to biopsy was close to 4 years post-transplant in the group of patients with clinical AbMR. Moreover, a common indication for the biopsy was proteinuria, which is clearly associated with chronic AbMR. Previous studies have demonstrated that AbMR became more common in biopsy specimens obtained >1 year after transplant, whereas TCMR progressively disappeared over time [50].
Conclusions
To conclude, urinary CXCL10 predicts and is related with both cellular-mediated and antibody-mediated acute histological damage. The only variable clearly correlated with high urinary CXCL10 excretion levels is the presence of DSA, whereas immunosuppressive exposure was not associated. Urinary CXCL10 is strongly correlated with AbMR and TCMR, independent of the other variables currently used to monitor kidney transplant, especially renal function, and it is independent of confounding factors, such as urinary leukocyte count. Even in patients who are positive for DSA, high urinary CXCL10 levels suggest that the patient has increased inflammation in the graft. By noninvasively measuring urinary CXCL10, we obtain information about the process taking place inside the kidney graft, and we can therefore monitor its evolution. A multicenter, randomized, controlled, prospective study is ongoing to determine if the detection and treatment of subclinical rejection as detected by urinary CXCL10 improves kidney allograft outcomes and will help to clarify the role of urinary CXCL10 in monitoring kidney transplant recipients [51].
Figures
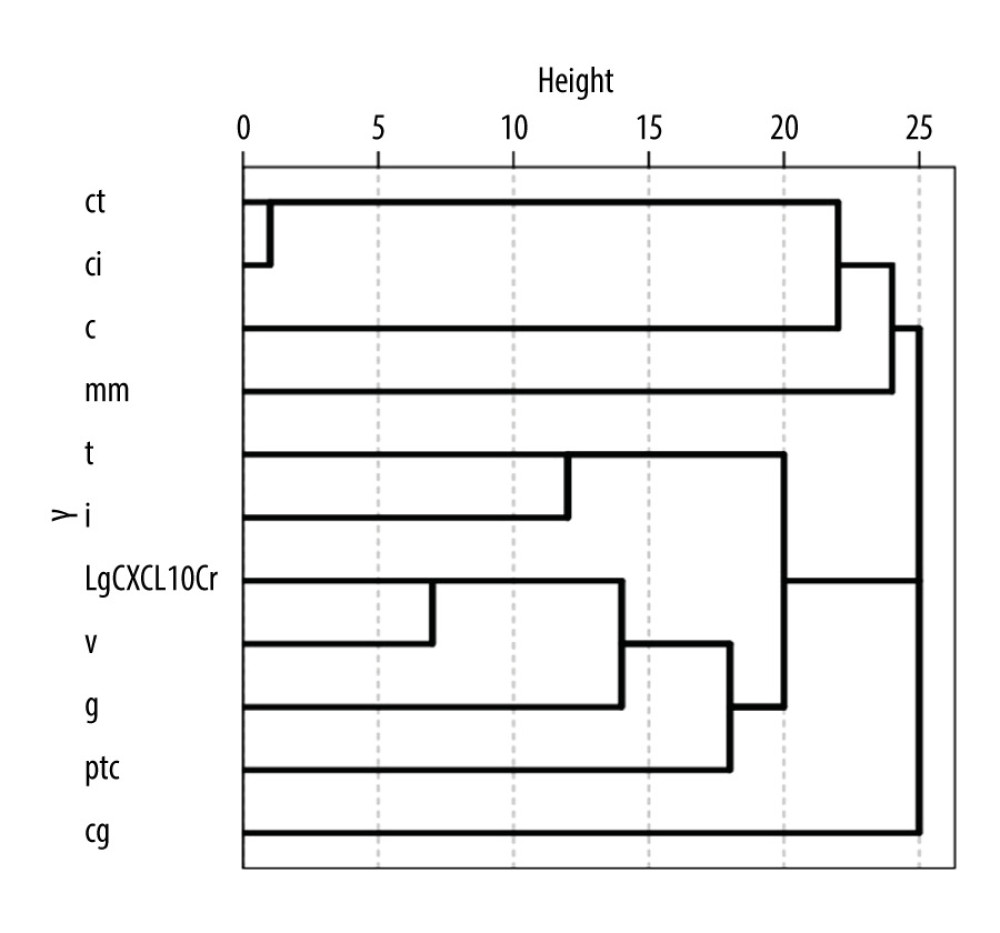 Figure 1. Dendrogram representation of unsupervised hierarchical clustering analysis of Banff scores and urinary CXCL10 excretion levels corrected by urine creatinine (CXCL10/Cr). The horizontal axis of the dendrogram represents the dissimilarity between clusters.
Figure 1. Dendrogram representation of unsupervised hierarchical clustering analysis of Banff scores and urinary CXCL10 excretion levels corrected by urine creatinine (CXCL10/Cr). The horizontal axis of the dendrogram represents the dissimilarity between clusters. 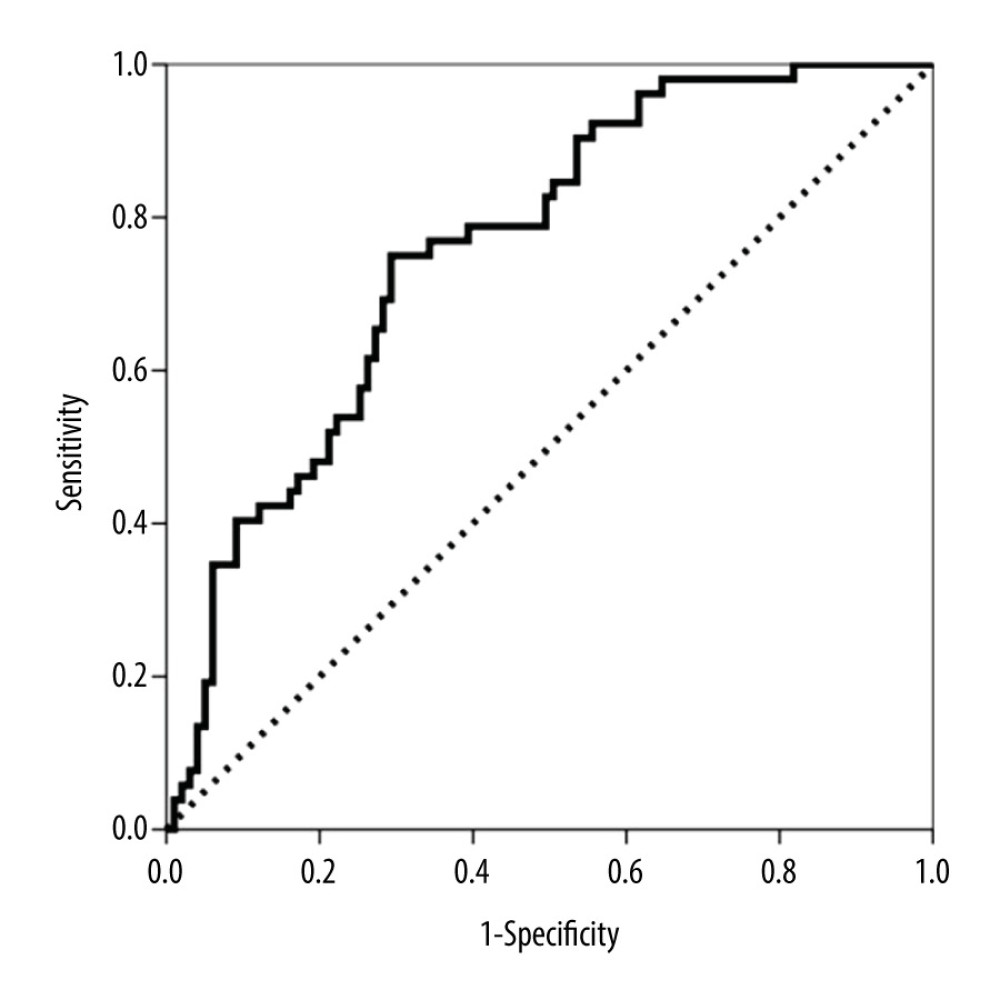 Figure 2. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy for predicting both clinical and subclinical antibody-mediated rejection (AbMR).
Figure 2. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy for predicting both clinical and subclinical antibody-mediated rejection (AbMR). 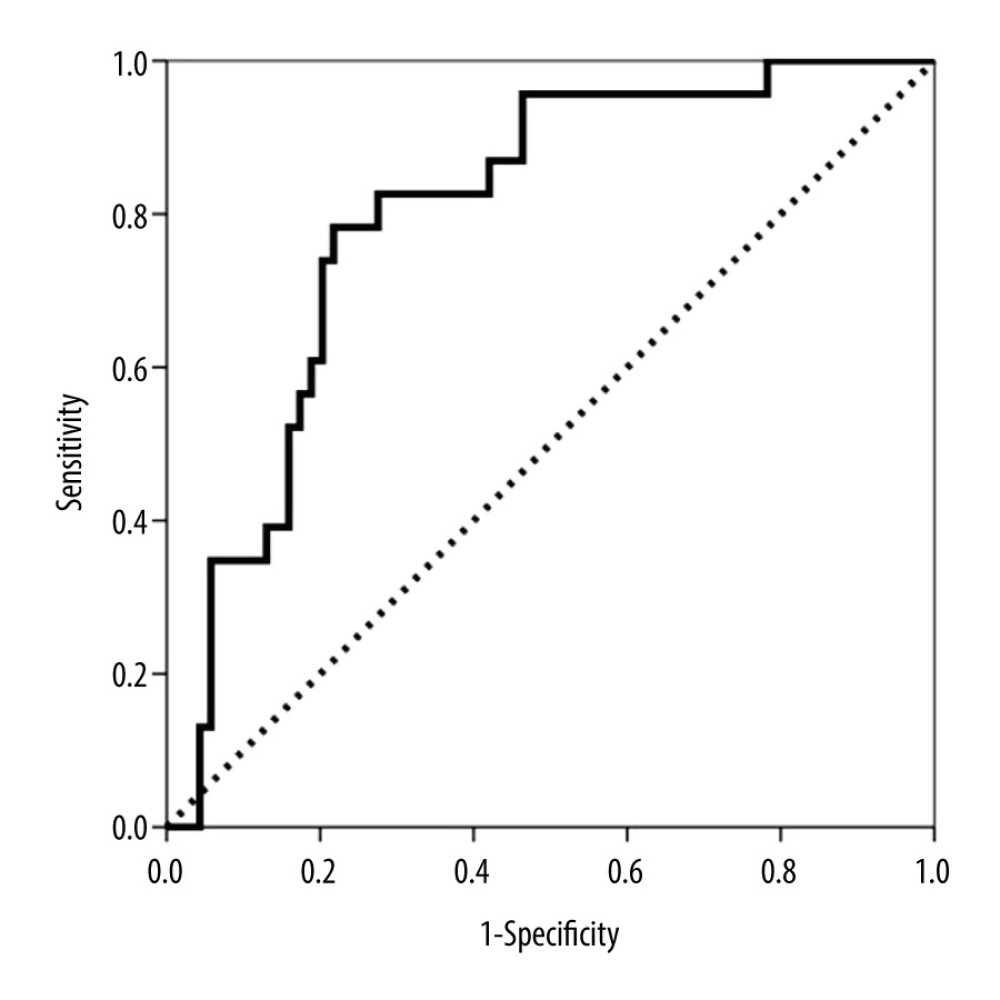 Figure 3. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy for predicting subclinical antibody-mediated rejection (AbMR).
Figure 3. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy for predicting subclinical antibody-mediated rejection (AbMR). 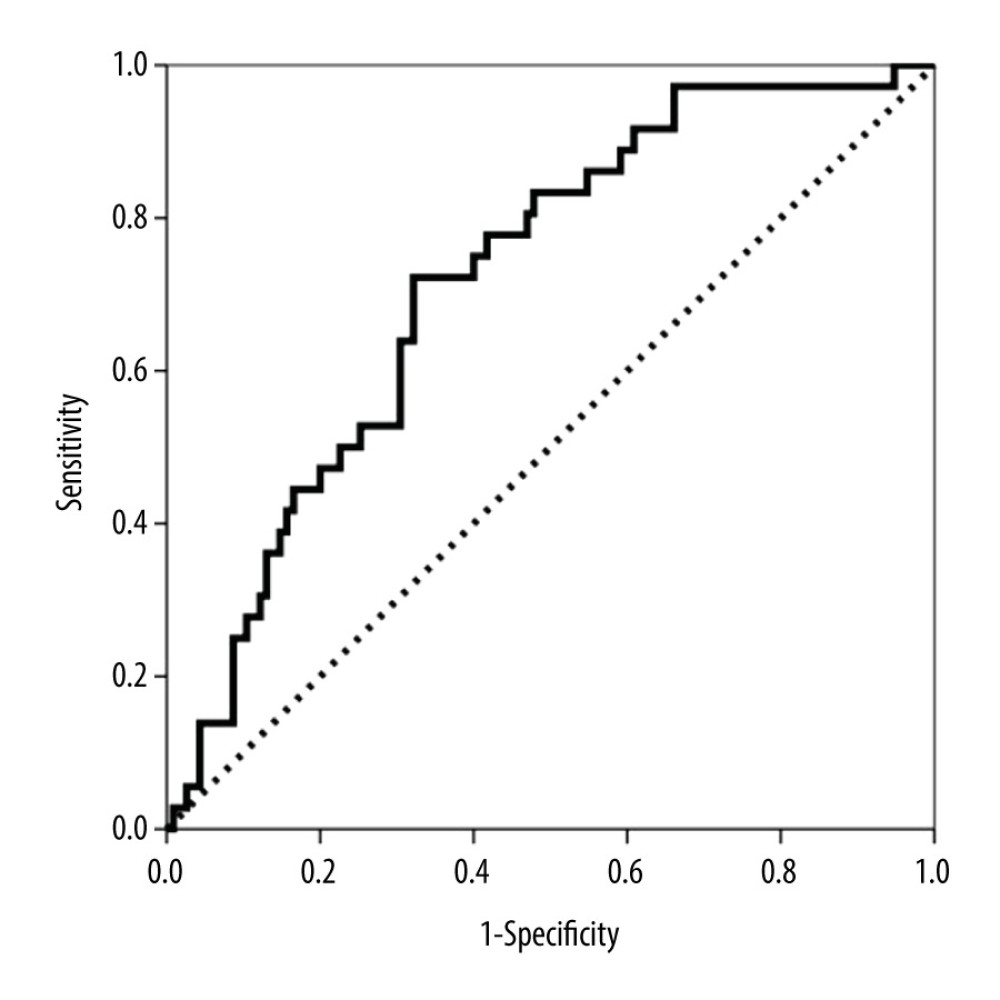 Figure 4. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy to discriminate between patients with and without clinical and subclinical T cell-mediated rejection (TCMR).
Figure 4. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy to discriminate between patients with and without clinical and subclinical T cell-mediated rejection (TCMR). 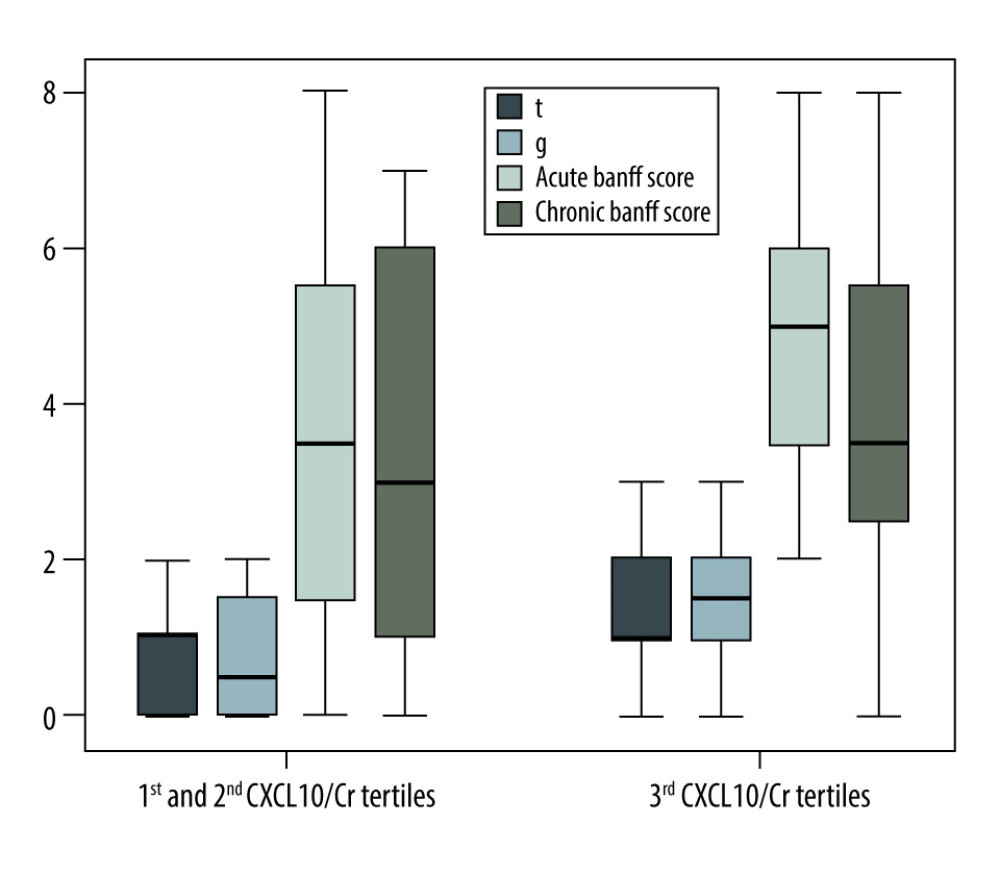 Figure 5. Differences among ‘t’ and ‘g’ scores, acute Banff score and chronic Banff scores comparing the third tertile versus first and second tertiles of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) in kidney transplant recipients with positive donor-specific antibodies.
Figure 5. Differences among ‘t’ and ‘g’ scores, acute Banff score and chronic Banff scores comparing the third tertile versus first and second tertiles of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) in kidney transplant recipients with positive donor-specific antibodies. 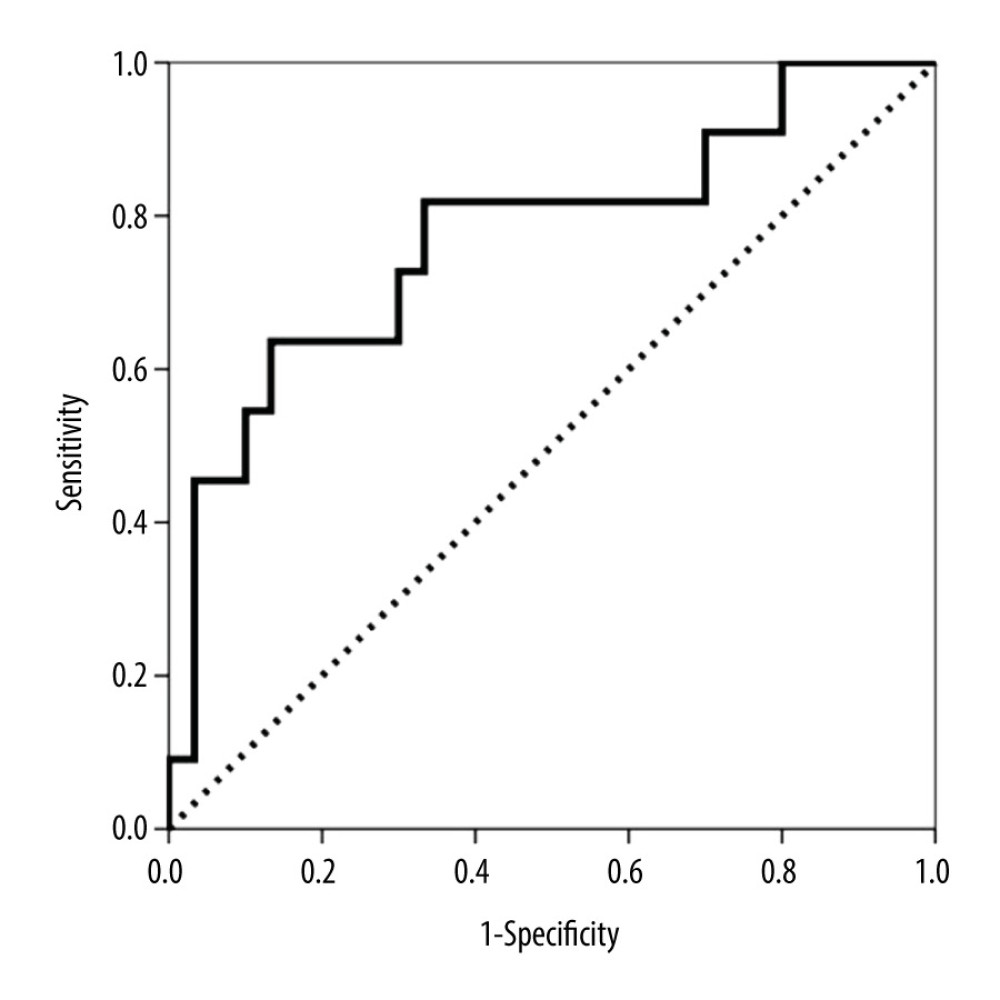 Figure 6. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at 6 months for predicting subclinical antibody-mediated rejection (AbMR) at the 1-year surveillance biopsy.
Figure 6. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at 6 months for predicting subclinical antibody-mediated rejection (AbMR) at the 1-year surveillance biopsy. 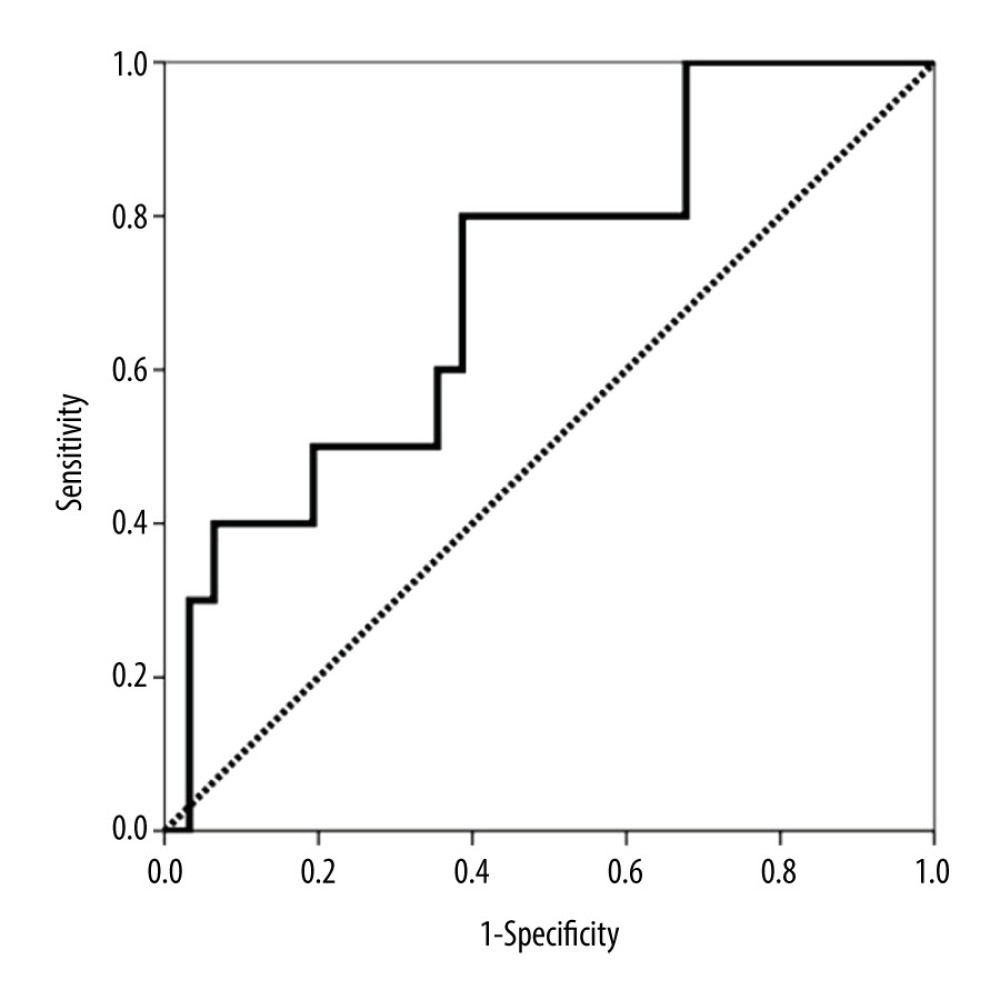 Figure 7. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at 6 months for predicting subclinical T cell-mediated rejection (TCMR) at the 1-year surveillance biopsy.
Figure 7. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at 6 months for predicting subclinical T cell-mediated rejection (TCMR) at the 1-year surveillance biopsy. Tables
Table 1. Main patient and transplant characteristics and variables related to both clinical and subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR).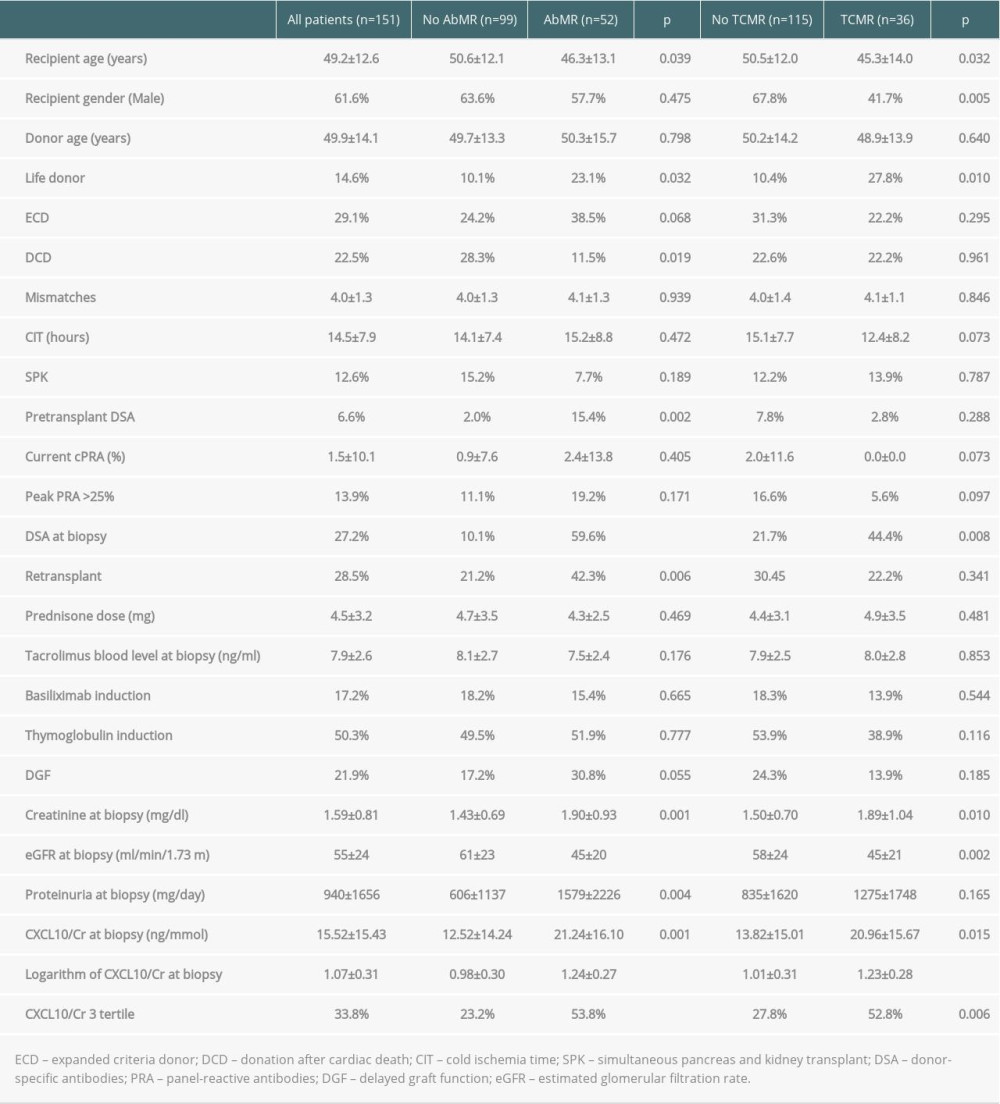 Table 2. Spearman correlation between Banff scores and logarithm of urinary CXCL10.
Table 2. Spearman correlation between Banff scores and logarithm of urinary CXCL10.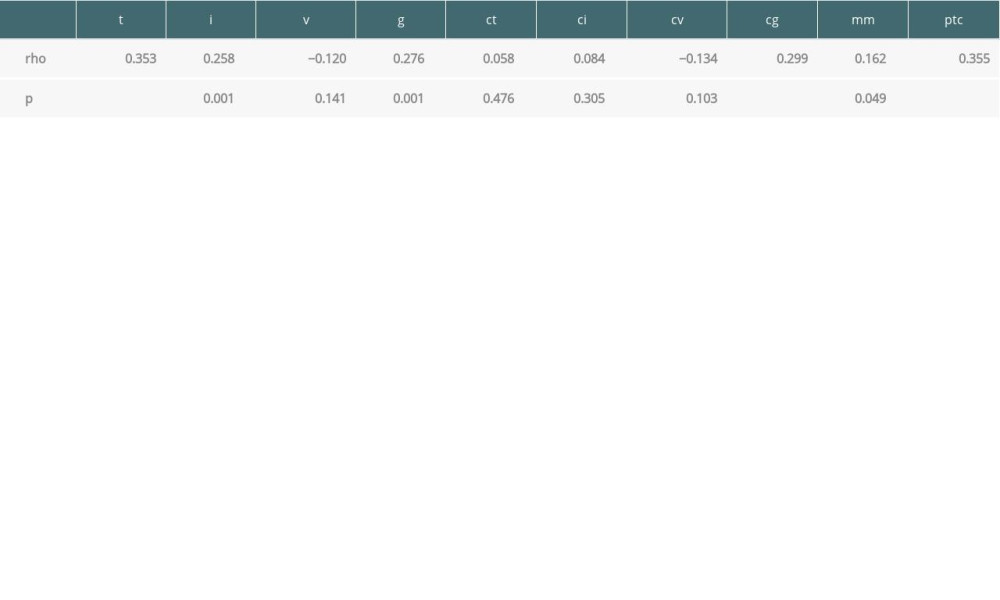 Table 3. Variables associated with the highest tertile of urinary CXCL10 excretion in surveillance biopsies.
Table 3. Variables associated with the highest tertile of urinary CXCL10 excretion in surveillance biopsies.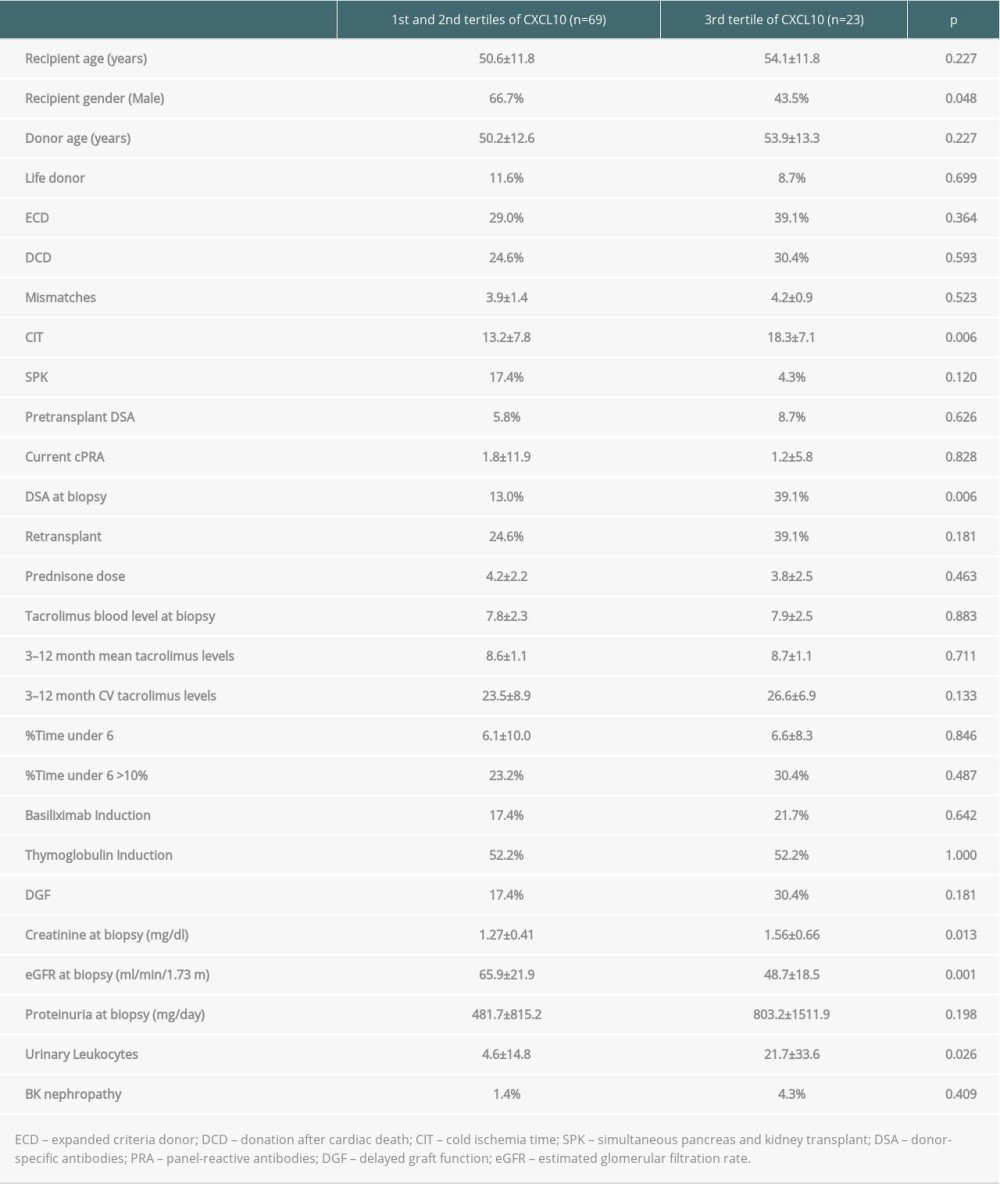 Table 4. Variables related to subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR).
Table 4. Variables related to subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR).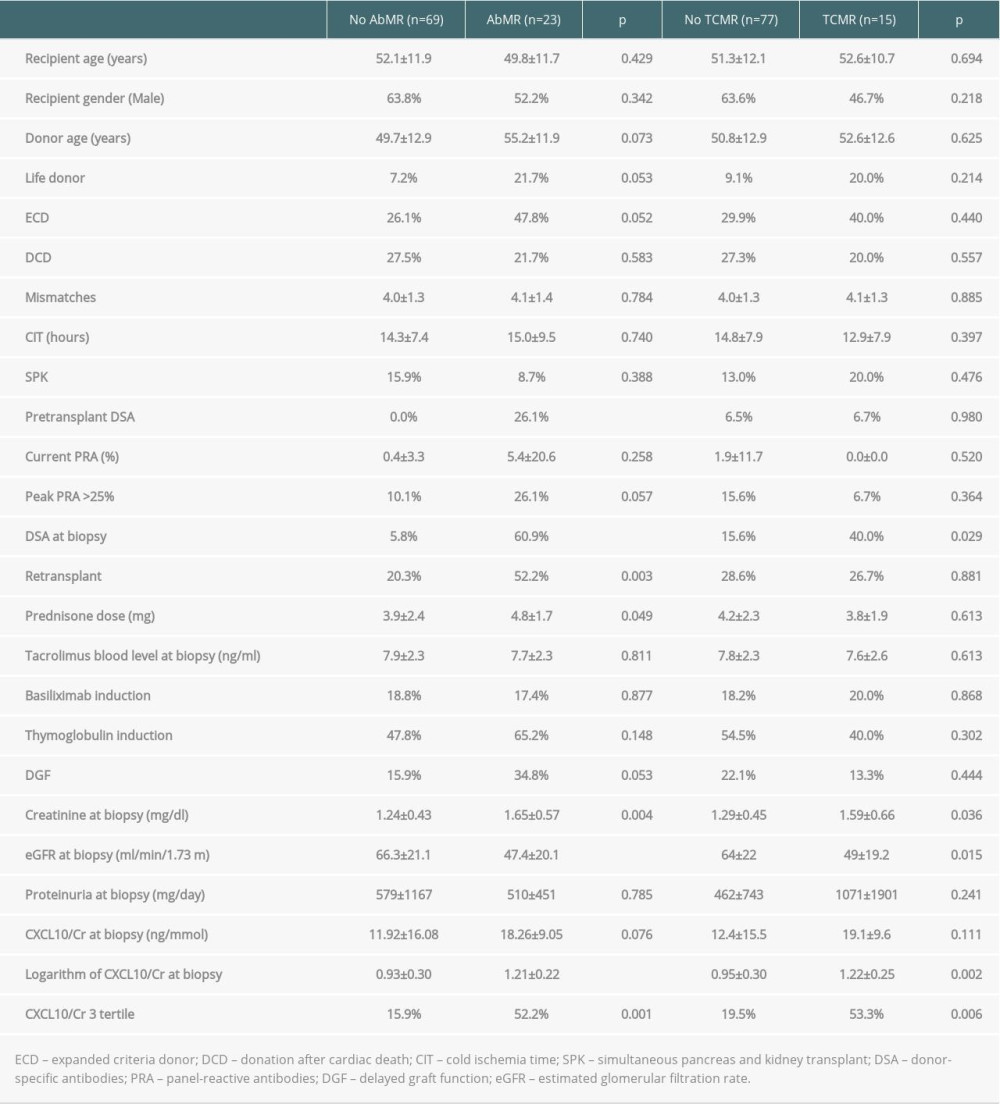
References
1. Knoll G, Muirhead N, Trpeski L, Patient survival following renal transplant failure in Canada: Am J Transplant, 2005; 5; 1719-24
2. Kaplan B, Meier-Kriesche H-U, Death after graft loss: An important late study endpoint in kidney transplantation: Am J Transplant, 2002; 2; 970-74
3. Lamb KE, Lodhi S, Meier-Kriesche HU, Long-term renal allograft survival in the United States: A critical reappraisal: Am J Transplant, 2011; 11(3); 450-62
4. El-Zoghby ZM, Stegall MD, Lager DJ, Identifying specific causes of kidney allograft loss: Am J Transplant, 2009; 9; 527-35
5. Sellarés J, de Freitas DG, Mengel M, Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence: Am J Transplant, 2012; 12; 388-99
6. Van Loon E, Senev A, Lerut E, Assessing the complex causes of kidney allograft loss: Transplantation, 2020; 104(12); 2557-66
7. Kidney Disease, Improving Global Outcomes (KDIGO) Transplant Work Group: KDIGO clinical practice guideline for the care of kidney transplant recipients: Am J Transplant, 2009; 9(Suppl 3); S1-155
8. Tait BD, Süsal C, Gebel HM, Consensus guidelines on the testing and clinical management issues associated with HLA and Non-HLA antibodies in transplantation: Transplantation, 2013; 95; 19-47
9. Rush D, Gibson IW, Subclinical inflammation in renal transplantation: Transplantation, 2019; 103; e139-45
10. Moreso F, Ibernon M, Gomà M, Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss: Am J Transplant, 2006; 6; 747-52
11. El Ters M, Grande JP, Keddis MT, Kidney allograft survival after acute rejection, the value of follow-up biopsies: Am J Transplant, 2013; 13; 2334-41
12. Loupy A, Vernerey D, Tinel C, Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts: J Am Soc Nephrol, 2015; 26; 1721-31
13. Orandi BJ, Chow EH, Hsu A, Quantifying renal allograft loss following early antibody-mediated rejection: Am J Transplant, 2015; 15; 489-98
14. Parajuli S, Joachim E, Alagusundaramoorthy S, Subclinical antibody-mediated rejection after kidney transplantation: Treatment outcomes: Transplantation, 2019; 103; 1722-29
15. Hirt-Minkowski P, De Serres SA, Ho J, Developing renal allograft surveillance strategies – urinary biomarkers of cellular rejection: Can J Kidney Health Dis, 2015; 2; 28
16. Veronese FV, Manfro RC, Roman FR, Reproducibility of the Banff classification in subclinical kidney transplant rejection: Clin Transplant, 2005; 19; 518-21
17. Erpicum P, Hanssen O, Weekers L, Non-invasive approaches in the diagnosis of acute rejection in kidney transplant recipients, part II: Omics analyses of urine and blood samples: Clin Kidney J, 2017; 10; 106-15
18. Hu H, Aizenstein BD, Puchalski A, Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction: Am J Transplant, 2004; 4; 432-37
19. Tatapudi RR, Muthukumar T, Dadhania D, Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP-10 and CXCR3 in urine: Kidney Int, 2004; 65; 2390-97
20. Matz M, Beyer J, Wunsch D, Early post-transplant urinary IP-10 expression after kidney transplantation is predictive of short- and long-term graft function: Kidney Int, 2006; 69; 1683-90
21. Peng W, Chen J, Jiang Y, Urinary fractalkine is a marker of acute rejection: Kidney Int, 2008; 74; 1454-60
22. Hu H, Kwun J, Aizenstein BD, Noninvasive detection of acute and chronic injuries in human renal transplant by elevation of multiple cytokines/chemokines in urine: Transplantation, 2009; 87; 1814-20
23. Schaub S, Nickerson P, Rush D, Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis: Am J Transplant, 2009; 9; 1347-53
24. Ho J, Rush DN, Karpinski M, Validation of urinary CXCL10 as a marker of borderline, subclinical, and clinical tubulitis: Transplantation, 2011; 92; 878-82
25. Jackson JA, Kim EJ, Begley B, Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection: Am J Transplant, 2011; 11; 2228-34
26. Hirt-Minkowski P, Amico P, Ho J, Detection of clinical and subclinical tubulo-interstitial inflammation by the urinary CXCL10 chemokine in a real-life setting: Am J Transplant, 2012; 12; 1811-23
27. Suthanthiran M, Schwartz JE, Ding R, Urinary-cell mRNA profile and acute cellular rejection in kidney allografts: N Engl J Med, 2013; 369; 20-31
28. Hricik DE, Nickerson P, Formica RN, Multicenter validation of urinary CXCL9 as a risk-stratifying biomarker for kidney transplant injury: Am J Transplant, 2013; 13; 2634-44
29. Hirt-Minkowski P, Ho J, Gao A, Prediction of long-term renal allograft outcome by early urinary CXCL10 chemokine levels: Transplant Direct, 2015; 1; 1
30. Rabant M, Amrouche L, Lebreton X, Urinary C-X-C motif chemokine 10 independently improves the noninvasive diagnosis of antibody-mediated kidney allograft rejection: J Am Soc Nephrol, 2015; 26; 2840-51
31. Blydt-Hansen TD, Gibson IW, Gao A, Elevated urinary CXCL10-to-creatinine ratio is associated with subclinical and clinical rejection in pediatric renal transplantation: Transplantation, 2015; 99; 797-804
32. Ho J, Sharma A, Mandal R, Detecting renal allograft inflammation using quantitative urine metabolomics and CXCL10: Transplant Direct, 2016; 19(2); e78
33. Rabant M, Amrouche L, Morin L, Early low urinary CXCL9 and CXCL10 might predict immunological quiescence in clinically and histologically stable kidney recipients: Am J Transplant, 2016; 16; 1868-81
34. Millán O, Budde K, Sommerer C, Urinary miR-155-5p and CXCL10 as prognostic and predictive biomarkers of rejection, graft outcome and treatment response in kidney transplantation: Br J Clin Pharmacol, 2017; 83; 2636-50
35. Ho J, Schaub S, Wiebe C, Urinary CXCL10 chemokine is associated with alloimmune and virus compartment-specific renal allograft inflammation: Transplantation, 2018; 102; 521-29
36. Ciftci HS, Tefik T, Savran MK, Urinary CXCL9 and CXCL10 levels and acute renal graft rejection: Int J Organ Transplant Med, 2019; 10; 53-63
37. Sigdel TK, Yang JYC, Bestard O, A urinary common rejection module (uCRM) score for non-invasive kidney transplant monitoring: PLoS One, 2019; 14; e0220052
38. Haas M, Loupy A, Lefaucheur C, The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials: Am J Transplant, 2018; 18; 293-307
39. Racusen LC, Solez K, Colvin RB, The Banff 97 working classification of renal allograft pathology: Kidney Int, 1999; 55(2); 713-23
40. Loupy A, Hill GS, Jordan SC, The impact of donor-specific anti-HLA antibodies on late kidney allograft failure: Nat Rev Nephrol, 2012; 8; 348-57
41. Halloran PF, Merino Lopez M, Barreto Pereira A, Identifying subphenotypes of antibody-mediated rejection in kidney transplants: Am J Transplant, 2016; 16; 908-20
42. Handschin J, Hirt-Minkowski P, Hönger G, Technical considerations and confounders for urine CXCL10 chemokine measurement: Transplant Direct, 2019; 6; e519
43. Wiebe C, Gibson IW, Blydt-Hansen TD, Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant: Am J Transplant, 2012; 12; 1157-67
44. Rodrigo E, Segundo DS, Fernandez Fresnedo G, Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development: Transplantation, 2015; 100; 2479-85
45. El Ters M, Grande JP, Keddis MT, Kidney allograft survival after acute rejection, the value of follow-up biopsies: Am J Transplant, 2013; 13; 2334-41
46. Davis S, Gralla J, Klem P, Tacrolimus intrapatient variability, time in therapeutic range, and risk of de novo donor-specific antibodies: Transplantation, 2020; 104; 881-87
47. Schinstock CA, Cosio F, Cheungpasitporn W, The value of protocol biopsies to identify patients with de novo donor-specific antibody at high risk for allograft loss: Am J Transplant, 2017; 17; 1574-84
48. Wiebe C, Gibson IW, Blydt-Hansen TD, Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody: Am J Transplant, 2015; 15; 2921-30
49. Gandolfini I, Harris C, Abecassis M, Rapid biolayer interferometry measurements of urinary CXCL9 to detect cellular infiltrates noninvasively after kidney transplantation: Kidney Int Rep, 2017; 2; 1186-93
50. Halloran PF, Chang J, Famulski K, Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients: J Am Soc Nephrol, 2015; 26; 1711-20
51. Ho J, Sharma A, Kroeker K, Multicentre randomised controlled trial protocol of urine CXCL10 monitoring strategy in kidney transplant recipients: BMJ Open, 2019; 9; e024908
Figures
 Figure 1. Dendrogram representation of unsupervised hierarchical clustering analysis of Banff scores and urinary CXCL10 excretion levels corrected by urine creatinine (CXCL10/Cr). The horizontal axis of the dendrogram represents the dissimilarity between clusters.
Figure 1. Dendrogram representation of unsupervised hierarchical clustering analysis of Banff scores and urinary CXCL10 excretion levels corrected by urine creatinine (CXCL10/Cr). The horizontal axis of the dendrogram represents the dissimilarity between clusters. Figure 2. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy for predicting both clinical and subclinical antibody-mediated rejection (AbMR).
Figure 2. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy for predicting both clinical and subclinical antibody-mediated rejection (AbMR). Figure 3. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy for predicting subclinical antibody-mediated rejection (AbMR).
Figure 3. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy for predicting subclinical antibody-mediated rejection (AbMR). Figure 4. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy to discriminate between patients with and without clinical and subclinical T cell-mediated rejection (TCMR).
Figure 4. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at biopsy to discriminate between patients with and without clinical and subclinical T cell-mediated rejection (TCMR). Figure 5. Differences among ‘t’ and ‘g’ scores, acute Banff score and chronic Banff scores comparing the third tertile versus first and second tertiles of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) in kidney transplant recipients with positive donor-specific antibodies.
Figure 5. Differences among ‘t’ and ‘g’ scores, acute Banff score and chronic Banff scores comparing the third tertile versus first and second tertiles of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) in kidney transplant recipients with positive donor-specific antibodies. Figure 6. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at 6 months for predicting subclinical antibody-mediated rejection (AbMR) at the 1-year surveillance biopsy.
Figure 6. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at 6 months for predicting subclinical antibody-mediated rejection (AbMR) at the 1-year surveillance biopsy. Figure 7. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at 6 months for predicting subclinical T cell-mediated rejection (TCMR) at the 1-year surveillance biopsy.
Figure 7. Area under the curve (AUC)-receiver operating characteristic (ROC) curve of urinary CXCL10 corrected by urine creatinine (CXCL10/Cr) at 6 months for predicting subclinical T cell-mediated rejection (TCMR) at the 1-year surveillance biopsy. Tables
 Table 1. Main patient and transplant characteristics and variables related to both clinical and subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR).
Table 1. Main patient and transplant characteristics and variables related to both clinical and subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR). Table 2. Spearman correlation between Banff scores and logarithm of urinary CXCL10.
Table 2. Spearman correlation between Banff scores and logarithm of urinary CXCL10. Table 3. Variables associated with the highest tertile of urinary CXCL10 excretion in surveillance biopsies.
Table 3. Variables associated with the highest tertile of urinary CXCL10 excretion in surveillance biopsies. Table 4. Variables related to subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR).
Table 4. Variables related to subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR). Table 1. Main patient and transplant characteristics and variables related to both clinical and subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR).
Table 1. Main patient and transplant characteristics and variables related to both clinical and subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR). Table 2. Spearman correlation between Banff scores and logarithm of urinary CXCL10.
Table 2. Spearman correlation between Banff scores and logarithm of urinary CXCL10. Table 3. Variables associated with the highest tertile of urinary CXCL10 excretion in surveillance biopsies.
Table 3. Variables associated with the highest tertile of urinary CXCL10 excretion in surveillance biopsies. Table 4. Variables related to subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR).
Table 4. Variables related to subclinical antibody-mediated rejection (AbMR) and T cell-mediated rejection (TCMR). In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








