09 April 2021: Original Paper
Hand-Assisted Laparoscopic Donor Nephrectomy in Living Donors with a History of Abdominal Surgery: A Retrospective Cohort Study
Takahisa Hiramitsu1ABCDEF*, Toshihide Tomosugi1B, Kenta Futamura1C, Manabu Okada1D, Norihiko Goto1E, Shunji Narumi1F, Kazuharu Uchida2F, Yoshihiko Watarai1DDOI: 10.12659/AOT.929752
Ann Transplant 2021; 26:e929752
Abstract
BACKGROUND: Hand-assisted laparoscopic donor nephrectomy (HALDN) is frequently performed in living kidney transplantation donors. This study investigated the efficacy and safety of HALDN for living donors with abdominal surgical histories.
MATERIAL AND METHODS: A total of 573 living kidney donors underwent donor nephrectomies for living donor kidney transplantation between January 2008 and May 2015. Eighteen donors underwent open donor nephrectomy and were excluded from analyses. Left HALDN was performed in 533 donors, including 44 donors with abdominal surgical histories and 489 donors without abdominal surgical histories. Right HALDN was performed in 22 donors, including 11 donors with abdominal surgical histories and 11 donors without abdominal surgical histories. Graft quality including the lengths of arteries, veins and ureters, time to initial urination, recipient complications, and recipient estimated glomerular filtration rate (eGFR) and operation quality including warm ischemic time, blood loss, operation duration, donor complications and donor eGFR, were compared between donors with and without abdominal surgical histories in the left and right HALDN groups.
RESULTS: The metrics of graft and operation quality were similar between living kidney donors with and without a history of abdominal surgery who underwent left or right HALDN.
CONCLUSIONS: The efficacy and safety of HALDN were not impaired by abdominal surgical histories.
Keywords: Glomerular Filtration Rate, Hand-Assisted Laparoscopy, Kidney Transplantation, Living Donors, Postoperative Complications, Abdomen, Laparoscopy, Nephrectomy, Tissue and Organ Harvesting
Background
Since the first reports of laparoscopic donor nephrectomy and hand-assisted laparoscopic donor nephrectomy (HALDN), laparoscopic procedures have been widely performed because they are minimally invasive for donors [1,2]. HALDN results in less postoperative pain, a shorter hospital stay, and an earlier postoperative recovery of donors [3]. Given that a donor nephrectomy itself does not benefit the donors, the procedure must be safe and the graft quality must not be impaired. The usefulness and safety of HALDN compared with those of an open donor nephrectomy (ODN) has been well-reported [3–7], but the operative indications of HALDN for living donors with abdominal surgical histories are rarely reported. Abdominal surgical histories can be risk factors for a difficult operation not only for HALDNs but also for other laparoscopic procedures [8–11]. We retrospectively investigated 533 left and 22 right HALDNs that were performed during January 2008–May 2015 in a single center and evaluated the operation and graft qualities between HALDNs with and without abdominal surgical histories.
Material and Methods
ETHICS REVIEW:
The Institutional Review Board of Nagoya Daini Red Cross Hospital approved this study (Approval number 1068) in accordance with the guidelines published in the Declaration of Helsinki. All patients’ data were retrospectively collected from their medical records. As such, the need for informed consent was waived.
STUDY DESIGN (EVALUATION OF LEFT AND RIGHT HALDNS):
To evaluate the operation quality, we investigated the operative duration, blood loss, warm ischemic time, donor complications, and donor postoperative kidney function by estimated glomerular filtration rate (eGFR) in the left HALDN group and the right HALDN group. To evaluate the graft quality, we investigated the arterial, venous, and ureteric lengths, the time to initial urination, recipient complications, and postoperative graft functions evaluated by eGFR. The renal arterial and venous lengths were defined as the length from the kidney hilum, and the ureteric length was defined as the length from the lower pole. This retrospective cohort study was conducted in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.
DONORS:
We reviewed the clinical data and operative records from 573 living kidney donors who underwent donor nephrectomies during January 2008–May 2015 performed by the same surgical team in a single center. The mean observation period was 67.7±20.1 months. Of the 573 donors, 18 were initially selected as ODNs (8 left ODNs and 10 right ODNs). HALDNs were classified into 4 groups: group A (44 donors), left HALDN with abdominal surgical histories; group B (489 donors), left HALDN without abdominal surgical histories; group C (11 donors), right HALDN with abdominal surgical histories; and group D (11 donors), right HALDN without abdominal surgical histories (Figure 1).
DEFINITION OF DONORS WITH ABDOMINAL SURGICAL HISTORIES:
Donors with abdominal surgical histories involved abdominal operations in the same surgical sites, the details of which are shown in Table 1.
DECISION ON THE PROCUREMENT SIDE:
Tc-99 m diethylene-triamine-pentaacetate (DTPA) renal scan and an enhanced computed tomography (CT) scan were used for the evaluation of preoperative kidney functions and anatomical features. The procurement side of the donor kidney was decided according to the inferior kidney function with more than 10% discrepancy in a DTPA renal scan and anatomical kidney problems, like renal stones and renal arterial aneurysms. Left kidneys were chosen preferentially regardless of the number of arteries and veins [12–15]. In cases where the right kidney functions were inferior, right kidneys were instead selected [16].
STANDARD LEFT HALDN:
Under general anaesthesia, the donor was placed in the right lateral decubitus position. A 7.5-cm skin incision was made for the hand port device in the mid-epigastric region. A 12-mm trocar for the flexible endoscope was placed inferior to the umbilicus. A 12-mm trocar was placed in the left lower-quadrant mid-axillary line and a 5-mm trocar was placed in the left upper-quadrant axillary line for the endoscopic instruments. A tissue-sealing device was used throughout the procedure. The spleen and descending colon were mobilized enough to expose the left renal vein, adrenal vein, and left gonadal vein. The ureter was dissected with the left gonadal vein. After sealing and cutting the adrenal vein, the adrenal gland was detached from the kidney upper pole. The fat pad of the upper pole was cut and the kidney was mobilized to expose the renal artery and vein. Lumbar veins were often divided to expose the renal artery and vein completely. The ureter and gonadal vein were divided in the caudal side after clipping. The kidney was put in a sterilized plastic bag and the renal artery and vein were divided with an endostapling device (Endo GIA™ Universal; United States Surgical, Norwalk, CT, USA). The kidney was taken through the hand port. After checking for bleeding, a drain was inserted from the 5-mm trocar site and the other wounds were closed.
STANDARD RIGHT HALDN:
Under general anaesthesia, the donor was placed in the left lateral decubitus position. A 7.5-cm lower-quadrant pararectal incision was made for the hand port device. A 12-mm trocar was placed inferior to the umbilicus for the flexible endoscope. A 12-mm trocar was placed in the mid-epigastric region and a 5-mm trocar was placed inferior to the xiphisternum for the endoscopic instruments. The ascending colon was mobilized to expose the ureter and inferior vena cava from the pararectal incision. The liver was gently mobilized with the endoscopic grasper from the 5-mm trocar. The inferior vena cava and renal vein were exposed after mobilization of the duodenum. Fat pads around the kidney were cut with a sealing device and the kidney was mobilized. From the dorsal side, the renal artery and vein were separated. The ureter was divided after clipping the caudal side and the renal artery and vein were divided with the endostapling device. The kidney was removed through the hand port. After checking for bleeding, a drain was inserted 5 mm from the trocar site and the other wounds were closed.
HALDN FOR DONORS WITH ABDOMINAL SURGICAL HISTORIES:
For a left HALDN, a 12-mm trocar was placed in the left lower-quadrant mid-axillary line prior to the handport to observe the intra-abdominal adhesion. In cases where adhesion at the handport site was identified, an adhesiotomy was performed after placing 12-mm trocars inferior to the umbilicus or a 5-mm trocar in the left upper-quadrant axillary line. After an adhesiotomy, the handport was placed and the rest of the surgical procedures were performed as a standard left HALDN. Adhesion that was not at the handport site could easily be detached in the process of a standard left HALDN.
For a right HALDN, most of the adhesiotomy was achieved via the 7.5-cm lower-quadrant pararectal incision. A right HALDN was performed with almost the same surgical procedures as a standard HALDN, regardless of abdominal surgical history.
STATISTICAL ANALYSIS:
A
Results
PARTICIPANTS:
A total of 573 consecutive living kidney transplants were performed during January 2008–May 2015. The mean observation period was 67.7±20.1 months. Of the 573 donors, 18 were initially selected as ODNs (8 left ODNs and 10 right ODNs). Four of the left ODNs and 2 of the right ODNs had abdominal surgical histories. Four of the left ODNs were suspected of having severe intra-abdominal adhesions, and 2 donors had undergone a total gastrectomy with a Roux-en-Y for early-stage gastric cancer. One donor had undergone a primary closure for a bladder rupture and ruptured duodenal ulcer due to a car accident. One donor had undergone a hysterectomy followed by adjuvant radiation therapy for cervical cancer. Two of the right ODNs had undergone an aortic replacement for an abdominal aortic aneurism and a ureterolithotomy. The remaining 4 left ODNs and 8 right ODNs were expected to have longer arteries and veins for arterial reconstruction due to having more than 4 arterial and renal arterial aneurisms and a feasible anastomosis for severe arterial calcification of the recipients (Figure 1). Of the 573 donors, 533 were selected as left HALDNs and 22 were selected as right HALDNs.
HALDNs were classified into 4 groups: group A (44 donors), left HALDN with abdominal surgical histories; group B (489 donors), left HALDN without abdominal surgical histories; group C (11 donors), right HALDN with abdominal surgical histories; and group D (11 donors), right HALDN without abdominal surgical histories (Figure 1). The details of surgical histories at the surgical site are shown in Table 1. In the left HALDN group, gynecological operations, including 22 hysterectomies, 9 caesarean sections, and 3 ovariectomies, gastrointestinal operations including 5 distal gastrectomies, 4 partial gastrectomies, and adhesiotomy, were identified. In the right HALDN group, gynecological operations, including 2 hysterectomies and 2 caesarean section, and 8 appendectomies, were identified. More than 2 operations were identified in 6 donors in left HALDN and in 1 donor in right HALDN.
DESCRIPTIVE DATA:
Donor and recipient characteristics are presented in Table 2. Significant differences were not identified between donor and recipient characteristics in the left and right HALDN groups, although there were significantly more males in group B than in group A. The surgical histories of groups A and C are shown in Table 1.
OUTCOME DATA:
In the left HALDN group (groups A and B), the mean operative duration, blood loss, and warm ischemic time were 212.1 min vs 222.6 min (P=0.152, 95% CI; −3.902–25.009), 37.1 ml vs 33.9 ml (P=0.702, 95% CI; 12.952–11.989) and 131.3 s vs 134.4 s (P=0.636, 95% CI; −9.917–16.219), respectively. One donor in group B had an intraoperative complication of uncontrollable bleeding in the renal vein (0.20%) that led to an open conversion. The donor was procured safely after the open conversion and discharged from hospital without any transfusion and postoperative complications. The most common postoperative complication in donors was wound infection, observed in 9 donors (20.5%) in group A and 59 donors (12.1%) in group B (P=0.152, risk ratio 1.066, 95% CI; 0.968–1.174). One donor in group B had postoperative bleeding (0.20%) that required a reoperation; it was treated laparoscopically without transfusion. After discharge, 2 small bowel obstructions caused by adhesion in the handport site and 2 incisional hernias (0.41% each) were identified in group B (Table 3).
The graft weight, arterial, venous, and ureteric lengths were 167.2 g vs 178.2 g (P=0.037, 95% CI; 0.706–21.296), 24.2 mm vs 25.7 mm (P=0.084, 95% CI; −0.199–3.195), 22.1 mm vs 23.5 mm (P=0.153, 95% CI; 0.501–3.204), and 103.8 mm vs 108.8 mm (P=0.331, 95% CI; −5.122–15.187), respectively (Table 3).
The results of recipient operations are shown in Table 4. The total ischemia time and time to initial urination were 83.1 min vs 101.1 min (P<0.001, 95% CI; 9.348–26.434) and 17.1 min vs 20.3 min (P=0.316, 95% CI; −3.019–9.337), respectively. Only 1 delayed graft function (0.20%) was identified in group B because of mistakenly sealing the renal artery with the tissue-sealing device before division with the endostapling device; the recipient needed 1 course of haemodialysis after the transplant. Ureteric leakages were identified in 3 recipients in group A (6.82%) and 3 recipients in group B (0.61%) (P<0.001, 95% CI; 9.348–26.434).
There was no significant difference in mean eGFR of the donors and recipients throughout the perioperative and postoperative periods (Figure 2A, 2B).
In the right HALDN group (groups C and D), the operative duration, blood loss, and warm ischemic time were 233.5 min vs 224.5 min (P=0.578, 95% CI; −9.000–15.903), 41.8 ml vs 184.6 ml (P=0.28, 95% CI; 142.727–128.630), and 171.0 s vs 160.8 s (P=0.545, 95% CI; −10.182–16.535), respectively. One donor in group D had an intraoperative complication of uncontrollable bleeding in the renal vein (9.0%) that led to an open conversion. However, the donor was procured safely without transfusion after an open conversion and was discharged from hospital without any postoperative complications. Two donors in group C had wound infections (18.2%) postoperatively. After discharge, 1 incisional hernia (9.0%) was identified in groups C and D. Graft weight, arterial, venous, and ureteric lengths were 160.3 g vs 175.0 g (P=0.283, 95% CI; 14.646–13.271), 31.6 mm vs 26.7 mm (P=0.175, 95% CI; −4.909–3.488), 18.2 mm vs 15.6 mm (P=0.263, 95% CI; −2.545–2.212), and 98.9 mm vs 109.5 mm (P=0.329, 95% CI; 10.591–10.596), respectively (Table 3). The results of the recipient’s operations are shown in Table 4. Total ischemia time and time to initial urination were 116.0 min vs 124.7 min (P=0.617, 95% CI; −8.727–17.197) and 22.1 min vs 17.7 min (P=0.578, 95% CI; −4.336–7.666), respectively. There was no delayed graft function in the right HALDN group.
Significant differences were not identified in the mean eGFR of the donors and recipients throughout the perioperative and postoperative periods (Figure 3A, 3B).
Discussion
Our analysis shows the efficacy and safety of left or right HALDN in living donors with abdominal surgical histories at the surgical sites. We performed 44 left HALDNs and 11 right HALDNs with abdominal surgical histories. Significant differences were not identified in the operation and graft quality for either the left or the right HALDN group. Graft quality was rarely evaluated by arterial, venous, and ureteric lengths. Longer arteries and veins are useful for safe anastomosis. Compared with the ODN, arterial and venous length tended to be shorter due to the wide endostaple. Arteries and veins should be exposed as safely as possible and as long as possible, especially in HALDNs. Short arteries and veins decrease the flexibility and lead to a difficult anastomosis, and a short vein might be a risk factor for graft venous thrombosis [12]. On the other hand, sufficient exposure of arteries and veins is considered difficult in living donors with abdominal surgical histories due to adhesion. However, in this study, arterial and venous lengths were not significantly different in a left and right HALDN, regardless of abdominal surgical histories. Further, it might lead to no significant difference in postoperative arterial and venous complication rates in the recipients. The significant difference identified in the graft weight in a left HALDN may have been because there were significantly more males in group B.
Based on recipient operation results, the total ischemic time was significantly longer in group B compared with group A. These results did not correlate with the abdominal surgical histories, but might correlate with the arterial numbers of grafts and their reconstructions. The numbers of grafts with more than 2 arteries and the arterial reconstructions were higher in group B compared with group A, although no significant difference was identified. Arterial reconstructions were associated with a significantly longer total ischemic time [15].
Many articles have referred to the incidence of urinary complications (1.0–9.2%) after kidney transplantation [17–21]. Common causes of urinary complications were vascularity of the donor ureter and the surgical technique. An appropriate ureter length is necessary for safe ureterovesical anastomosis. Some articles recommended a shorter ureter because of distal ureteric ischemia and kinking [22]. However, tension in the ureterovesical anastomosis site due to a ureter that is too short might cause urine leakage and stricture. In the procurement procedure, the ureter should be procured as long as possible, and after the reperfusion of the graft it should be cut to the appropriate length according to the bladder size and vascularity of the distal ureter. On the other hand, in living donors with abdominal surgical histories, especially in the lower abdomen, gaining the appropriate ureteric length to maintain vascularity of the donor ureter is considered difficult due to adhesion. This study demonstrated that in a right HALDN, abdominal surgical histories did not increase ureteric complication rates. However, in a left HALDN, ureteric leakage was observed more frequently in the recipients who received transplants from donors with abdominal surgical histories. These ureteric leakages were all treated with re-anastomosis. The intraoperative findings of these recipients did not show necrosis of the ureter, and all ureteric leakages were considered to be due to inappropriate anastomosis. None of these ureteric leakages were directly correlated with abdominal surgical histories because no significant difference in the ureteric length was identified between donors with and without abdominal surgical histories. The adhesion around the ureter was not identified in the donors with abdominal surgical histories. Although other various complications were identified in recipients, these were treated well and there was no significant difference in any complication rate for a left and right HALDN.
The eGFR was investigated to evaluate graft functions and remaining kidney functions in both recipients and donors. There was no significant difference in the mean eGFR of recipients and donors throughout all perioperative and postoperative periods. This demonstrated that procurements for transplantation were performed efficiently and did not cause any harm to the donor’s remaining kidney functions, even from donors with abdominal surgical histories.
The limitation of this study was that it was retrospective and 18 donors were initially selected for ODN. Prospective studies on the impact of abdominal histories at the surgical sites on HALDN are needed. HALDN for donors with a total gastrectomy with a Roux-en-Y reconstruction, hysterectomy followed by adjuvant radiation therapy, aortic replacement for the abdominal aortic aneurism, and a ureterolithotomy will be the next challenges.
Conclusions
Even in living donors with abdominal surgical histories at the surgical sites, left or right HALDN was performed efficiently and safely, except for donors who had undergone a total gastrectomy with a Roux-en-orY reconstruction, hysterectomy followed by adjuvant radiation therapy, aortic replacement for the abdominal aortic aneurism, and a ureterolithotomy.
Figures
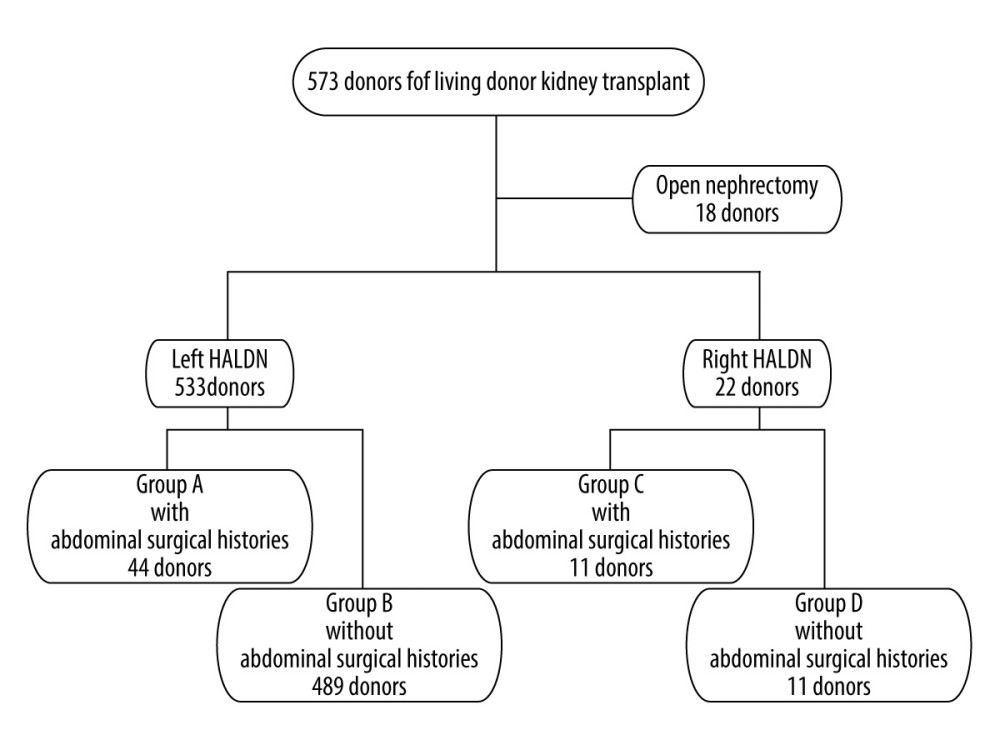 Figure 1. Patient flow chart. HALDN – hand-assisted laparoscopic donor nephrectomy.
Figure 1. Patient flow chart. HALDN – hand-assisted laparoscopic donor nephrectomy. 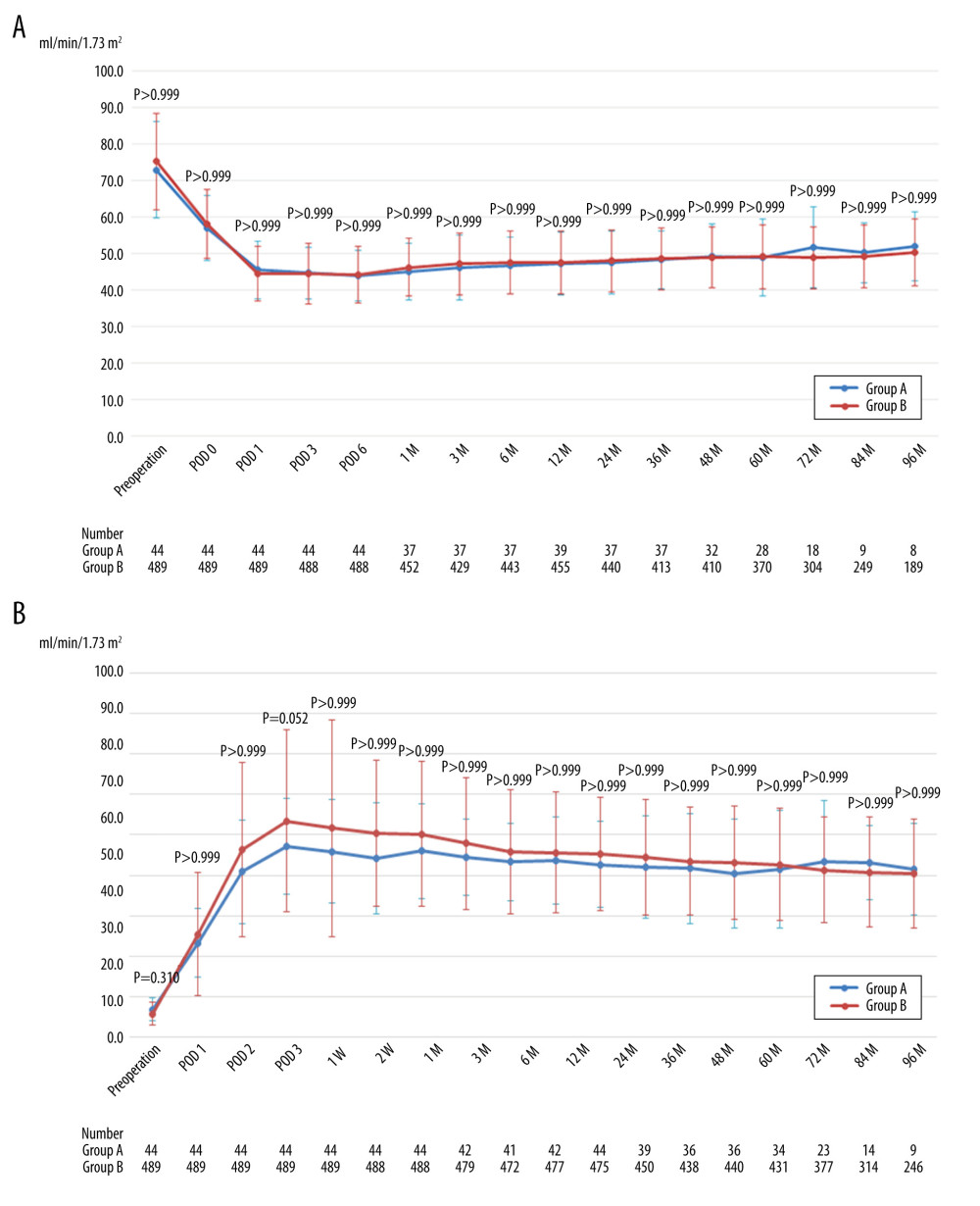 Figure 2. (A) Mean estimated glomerular filtration rate of donors after left hand-assisted laparoscopic donor nephrectomy. (B) Mean estimated glomerular filtration rate of recipients transplanted from left hand-assisted laparoscopic donor nephrectomy donors. M – months postoperatively; POD – postoperative day; The Bonferroni method was used to adjust for multiple comparisons.
Figure 2. (A) Mean estimated glomerular filtration rate of donors after left hand-assisted laparoscopic donor nephrectomy. (B) Mean estimated glomerular filtration rate of recipients transplanted from left hand-assisted laparoscopic donor nephrectomy donors. M – months postoperatively; POD – postoperative day; The Bonferroni method was used to adjust for multiple comparisons. 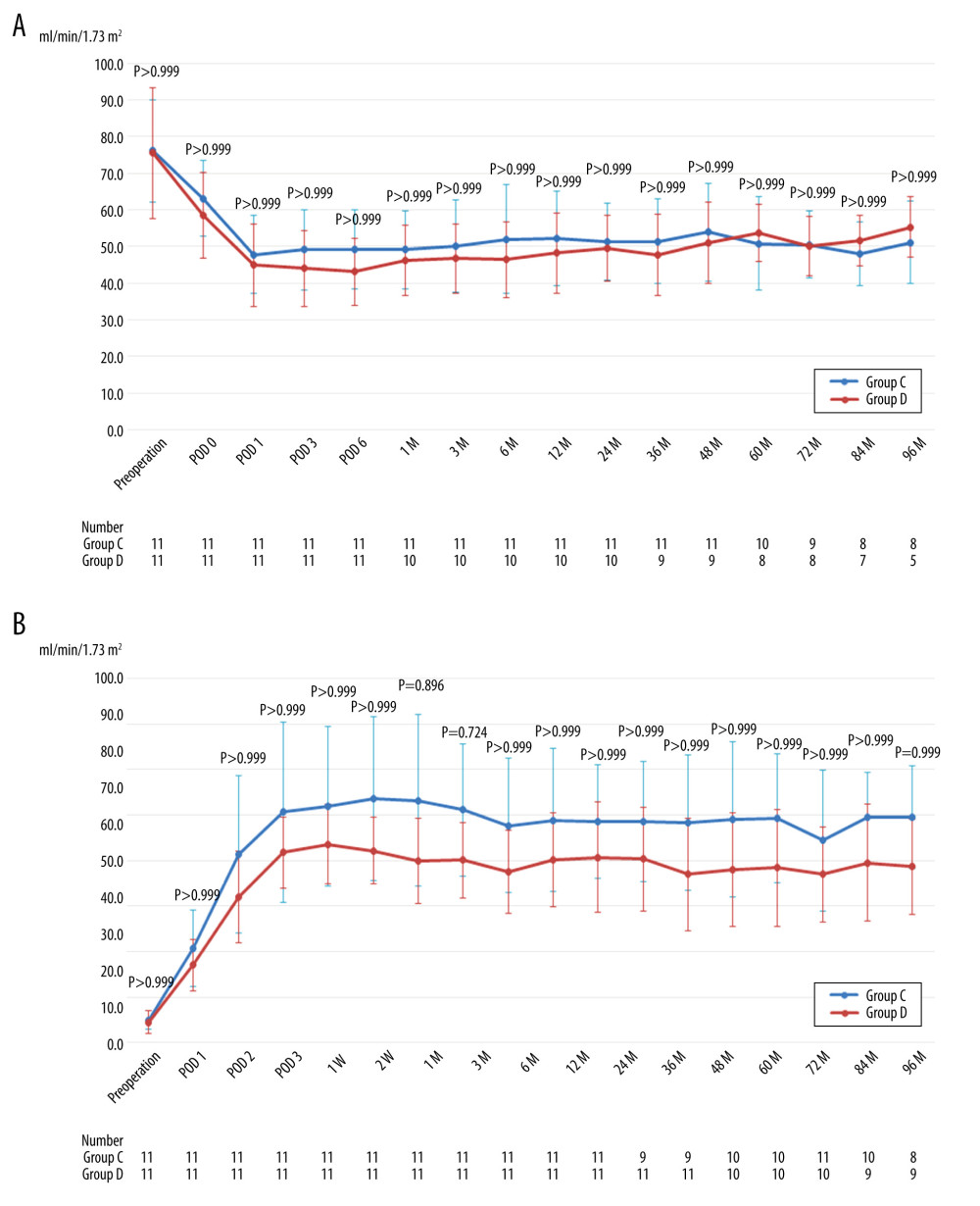 Figure 3. (A) Mean estimated glomerular filtration rate of donors after right hand-assisted laparoscopic donor nephrectomy. (B) Mean estimated glomerular filtration rate of recipients transplanted from right hand-assisted laparoscopic donor nephrectomy donors. M – months postoperatively; POD – postoperative day; The Bonferroni method was used to adjust for multiple comparisons.
Figure 3. (A) Mean estimated glomerular filtration rate of donors after right hand-assisted laparoscopic donor nephrectomy. (B) Mean estimated glomerular filtration rate of recipients transplanted from right hand-assisted laparoscopic donor nephrectomy donors. M – months postoperatively; POD – postoperative day; The Bonferroni method was used to adjust for multiple comparisons. References
1. Ratner LE, Ciseck LJ, Moore RG, Laparoscopic live donor nephrectomy: Transplantation, 1995; 60; 1047-49
2. Wolf JJ, Tchetgen M, Merion R, Hand-assisted laparoscopic live donor nephrectomy: Urology, 1998; 52; 885-87
3. Clayman RV, Kavoussi LR, Soper NJ, Laparoscopic nephrectomy: N Engl J Med, 1991; 324; 1370-71
4. Flowers JL, Jacobs S, Cho E, Comparison of open and laparoscopic live donor nephrectomy: Ann Surg, 1997; 226; 483-90
5. Su LM, Ratner LE, Montgomery RA, Laparoscopic live donor nephrectomy: Trends in donor and recipient morbidity following 381 consecutive cases: Ann Surg, 2004; 240; 358-63
6. Subramanian T, Dageforde LA, Vachharajani N, Mini-incision versus hand-assisted laparoscopic donor nephrectomy in living-donor kidney transplantation: A retrospective cohort study: Int J Surg, 2018; 53; 339-44
7. Fonouni H, Mehrabi A, Golriz M, Comparison of the laparoscopic versus open live donor nephrectomy: An overview of surgical complications and outcome: Langenbecks Arch Surg, 2014; 399; 543-51
8. Nakajima I, Iwadoh K, Koyama I, Nine-yr experience of 700 hand-assisted laparoscopic donor nephrectomies in Japan: Clin Transplant, 2012; 26; 797-807
9. Barleben A, Gandhi D, Nquyen XM, Is laparoscopic colon surgery appropriate in patients who have had previous abdominal surgery?: Am Surg, 2009; 75; 1015-19
10. Akyurek N, Salman B, Irkorucu O, Laparoscopic cholecystectomy in patients with previous abdominal surgery: JSLS, 2005; 9; 178-83
11. Seetahal S, Obirieze A, Cornwell EE, Open abdominal surgery: A risk factor for future laparoscopic surgery?: Am J Surg, 2015; 209; 623-26
12. Mandal AK, Cohen C, Montgomery RA, Should the indications for laparoscopic live donor nephrectomy of the right kidney be the same as for the open procedure? Anomalous left renal vasculature is not contraindication to laparoscopic left donor nephrectomy: Transplanatation, 2001; 71; 660-64
13. Sagban TA, Baur B, Rump LC, Long-term graft outcome after renal arterial reconstruction during living related kidney transplantation: Langenbecks Arch Surg, 2014; 399; 441-47
14. Makiyama K, Tanabe K, Ishida H, Successful renovascular reconstruction for renal allografts with multiple renal arteries: Transplantation, 2003; 75; 828-32
15. Hiramitsu T, Futamura K, Okada M, Impact of arterial reconstruction with recipient’s own internal iliac artery for multiple graft arteries on living donor kidney transplantation: Medicine 43, 2015; 94(43); e1811
16. Wang K, Zhang P, Xu X, Right versus left laparoscopic living-donor nephrectomy: A meta-analysis: Exp Clin Transplant, 2015; 13; 214-26
17. Mangus RS, Haag BW, Carter CB, Stented Lich-Gregoir ureteroneocystostomy: Case series report and cost-effectiveness analysis: Transplant Proc, 2004; 36; 2929
18. Shoskes DA, Hanbury D, Cranston D, Urological complications in 1,000 consecutive renal transplant recipients: J Urol, 1995; 153; 18
19. Streeter EH, Little DM, Cranston DW, The urological complications of renal transplantation: A series of 1535 patients: BJU Int, 2002; 90; 627-34
20. Choi YS, Kim KS, Choi SW, Ureteral complications in kidney transplantation: Analysis and management of 853 consecutive laparoscopic living-donor nephrectomies in a single center: Transplant Proc, 2016; 48; 2684-88
21. Bruintjes MHD, d’Ancona FCH, Zhu X, An update on early urological complications in kidney transplantation: A national cohort study: Ann Transplant, 2019; 24; 617-24
22. Butterworth PC, Horsburgh T, Veitch PS, Ureterovesical anastomosis in renal transplants: fewer complications with the extravesical technique: Transplant Proc, 1997; 29; 151
Figures
 Figure 1. Patient flow chart. HALDN – hand-assisted laparoscopic donor nephrectomy.
Figure 1. Patient flow chart. HALDN – hand-assisted laparoscopic donor nephrectomy. Figure 2. (A) Mean estimated glomerular filtration rate of donors after left hand-assisted laparoscopic donor nephrectomy. (B) Mean estimated glomerular filtration rate of recipients transplanted from left hand-assisted laparoscopic donor nephrectomy donors. M – months postoperatively; POD – postoperative day; The Bonferroni method was used to adjust for multiple comparisons.
Figure 2. (A) Mean estimated glomerular filtration rate of donors after left hand-assisted laparoscopic donor nephrectomy. (B) Mean estimated glomerular filtration rate of recipients transplanted from left hand-assisted laparoscopic donor nephrectomy donors. M – months postoperatively; POD – postoperative day; The Bonferroni method was used to adjust for multiple comparisons. Figure 3. (A) Mean estimated glomerular filtration rate of donors after right hand-assisted laparoscopic donor nephrectomy. (B) Mean estimated glomerular filtration rate of recipients transplanted from right hand-assisted laparoscopic donor nephrectomy donors. M – months postoperatively; POD – postoperative day; The Bonferroni method was used to adjust for multiple comparisons.
Figure 3. (A) Mean estimated glomerular filtration rate of donors after right hand-assisted laparoscopic donor nephrectomy. (B) Mean estimated glomerular filtration rate of recipients transplanted from right hand-assisted laparoscopic donor nephrectomy donors. M – months postoperatively; POD – postoperative day; The Bonferroni method was used to adjust for multiple comparisons. Tables
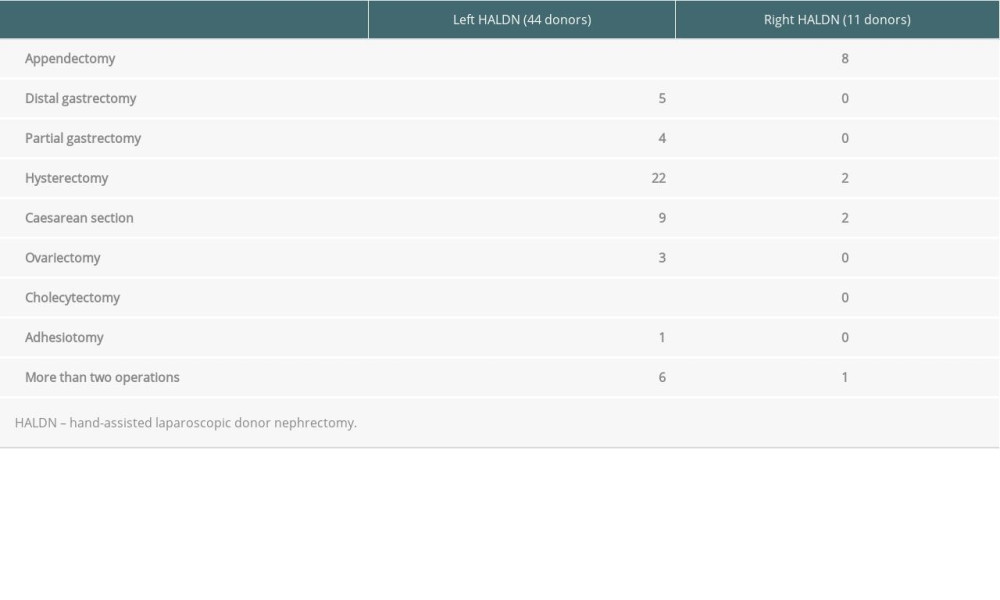 Table 1. The details of abdominal surgical histories of donors.
Table 1. The details of abdominal surgical histories of donors.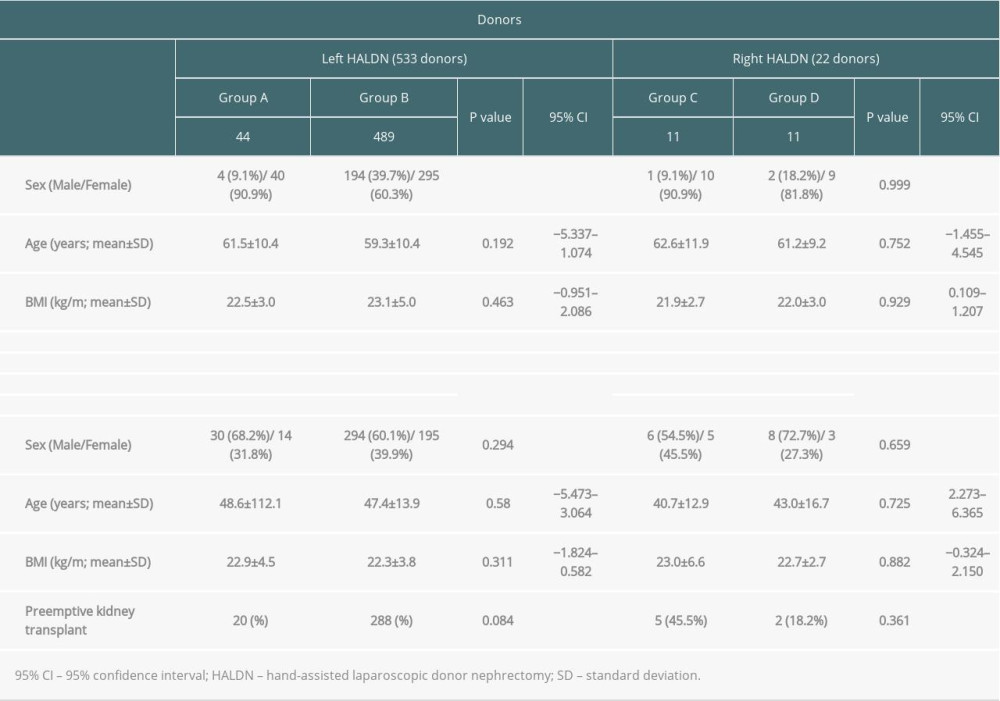 Table 2. Patient characteristics of donors and recipients.
Table 2. Patient characteristics of donors and recipients.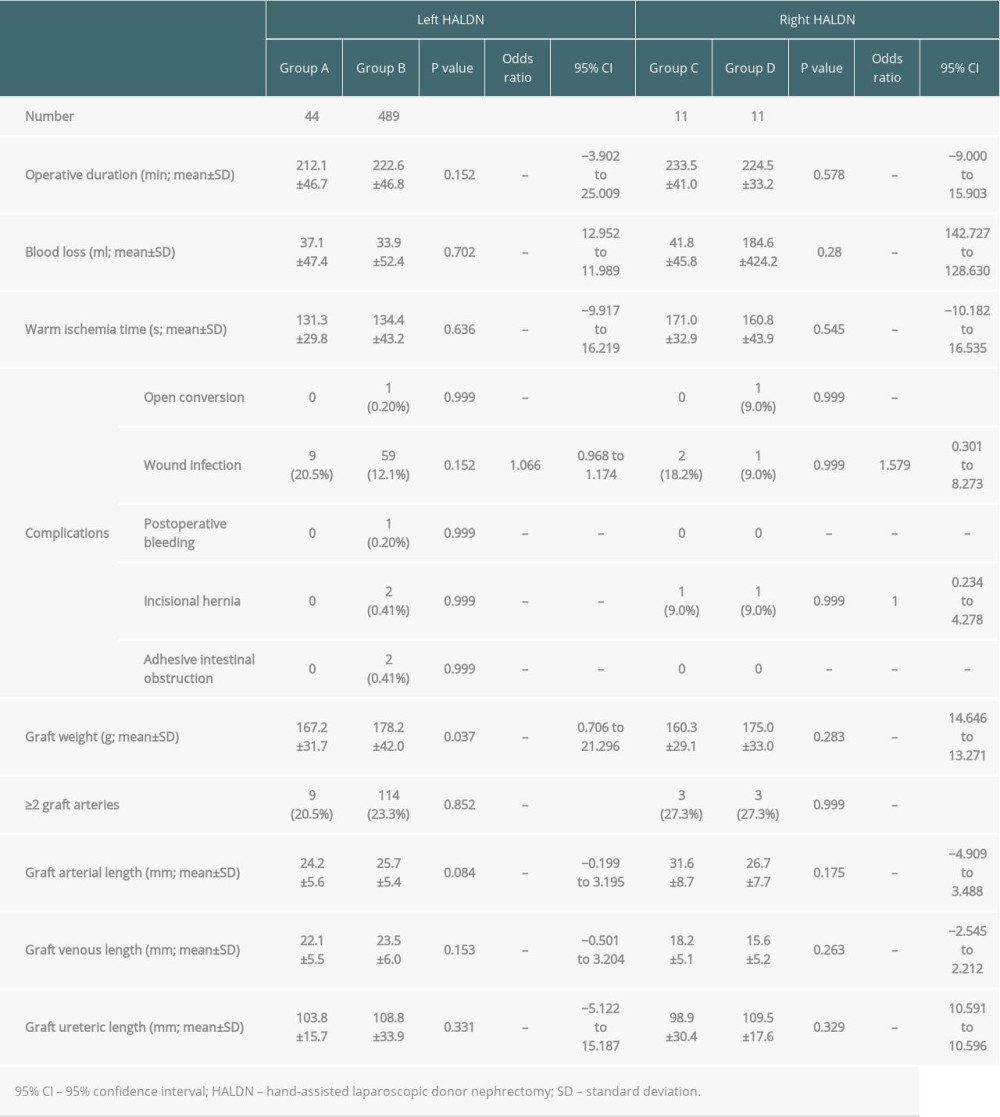 Table 3. Results of left and right HALDNs.
Table 3. Results of left and right HALDNs.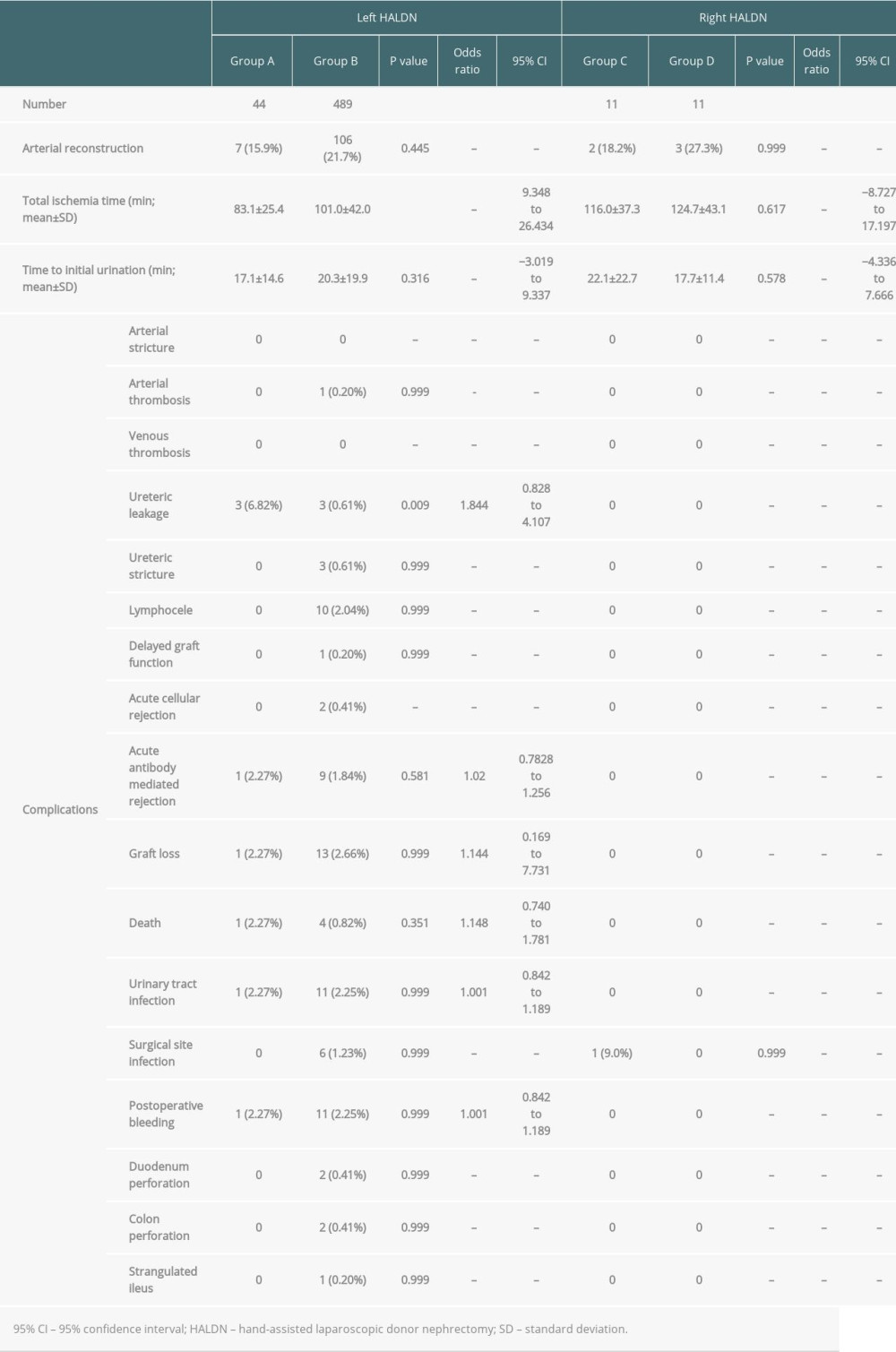 Table 4. Results of recipients.
Table 4. Results of recipients. Table 1. The details of abdominal surgical histories of donors.
Table 1. The details of abdominal surgical histories of donors. Table 2. Patient characteristics of donors and recipients.
Table 2. Patient characteristics of donors and recipients. Table 3. Results of left and right HALDNs.
Table 3. Results of left and right HALDNs. Table 4. Results of recipients.
Table 4. Results of recipients. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








