02 December 2022: Original Paper
Pure Laparoscopic Versus Open Right Hepatectomy in Living Liver Donors: Graft Weight Discrepancy
Jiwon Seo1ABCDEF, Suk Kyun Hong1ABCDEF*, Sola Lee1BE, Su young Hong1BE, YoungRok Choi1BE, Nam-Joon Yi1BE, Kwang-Woong Lee1BE, Kyung-Suk Suh1BDDOI: 10.12659/AOT.938274
Ann Transplant 2022; 27:e938274
Abstract
BACKGROUND: Accurate volumetric evaluation of donors’ livers before surgery is crucial for successful living-donor liver transplantation. However, there are few studies on the volumetric evaluation in the recently popularized pure laparoscopic donor hepatectomy method, in contrast to the number of studies for conventional donor hepatectomy. We aimed to analyze the difference between estimated graft weight and actual graft weight in pure laparoscopic donor right hepatectomy (PLDRH) and conventional donor right hepatectomy (CDRH) procedures.
MATERIAL AND METHODS: The medical records of 612 donors who underwent right hepatectomy in living-donor liver transplantation between January 2014 and December 2020 were retrospectively reviewed. The CDRH group targeted patients from January 2014 to October 2015, and the PLDRH group targeted patients from March 2016 to December 2020.
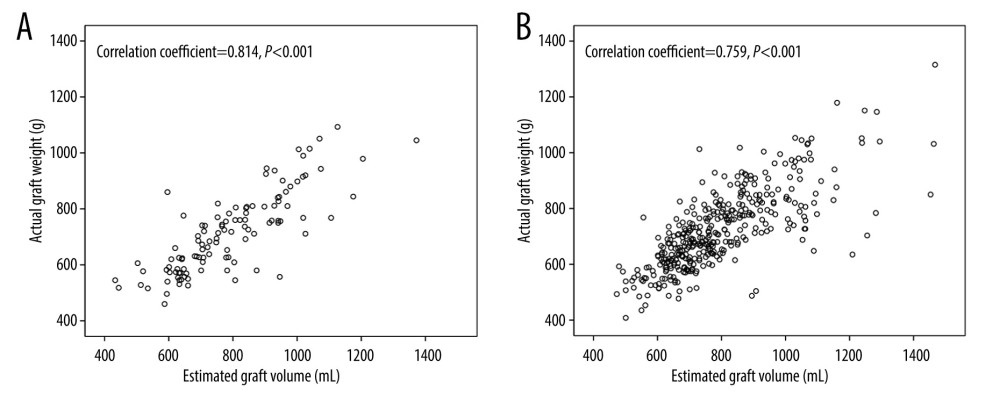
RESULTS: There were 119 and 376 donors who underwent CDRH and PLDRH, respectively. Although there was no significant difference in the estimated graft weights (P=0.994) and actual graft weights (P=0.489) between the groups, the estimated graft weights were significantly higher than the actual graft weights in both groups. However, the estimated graft weight and actual graft weight showed linear correlations in both the CDRH (r=0.81, P<0.001) and PLDRH (r=0.76, P<0.001) groups, with the CDRH group having greater linearity.
CONCLUSIONS: The estimates of graft weight were similar between the 2 groups. However, since the actual graft weight tended to be smaller in the PLDRH group, this should be considered before surgery.
Keywords: Hepatectomy, Laparoscopy, Liver Transplantation, Living Donors, Humans, Liver
Background
Liver transplantation is currently considered the criterion standard treatment for patients with end-stage liver disease. The use of living-donor liver transplantation (LDLT) in particular is increasing worldwide owing to its advantages [1]. Its faster transplantation route has alleviated deficiencies in organs from deceased donors and reduced mortality in patients awaiting liver transplantation [1].
However, accurate volumetric determination, which is necessary for an accurate remnant liver volume and graft-to-recipient weight ratio (GRWR), is crucial for the safety of both the donors and recipients [2]. While a remnant liver volume of 30–35% of the original volume is required for donor safety, recipients need at least 40% of the standard liver volume or a 0.8% GRWR [3].
With the recent increase in liver transplantation, as well as the development of new surgical techniques and increased experience among surgeons, pure laparoscopic donor hepatectomy (PLDH) has emerged to satisfy donors’ cosmetic and functional needs. As several studies have confirmed its feasibility and safety, more centers are beginning to use PLDH [4–6]. However, compared to conventional donor hepatectomy, little attention has been paid to volumetric evaluation in PLDH.
Since the initiation of PLDH program in November 2015, our center performed more than 400 cases of PLDH, most of which were right hepatectomies. The aim of this study was to analyze the difference between the estimated graft weight (EGW) and actual graft weight (AGW) in pure laparoscopic donor right hepatectomy (PLDRH), and to compare the correlation between the EGW and AGW with that in conventional donor right hepatectomy (CDRH). This may be one of the largest studies to compare graft weights between PLDRH and CDRH procedures.
Material and Methods
STUDY DESIGN AND PATIENTS:
This retrospective study analyzed graft weight discrepancies according to surgical methods in LDLT. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of the Seoul National University Hospital (IRB no. 2111-097-1272).
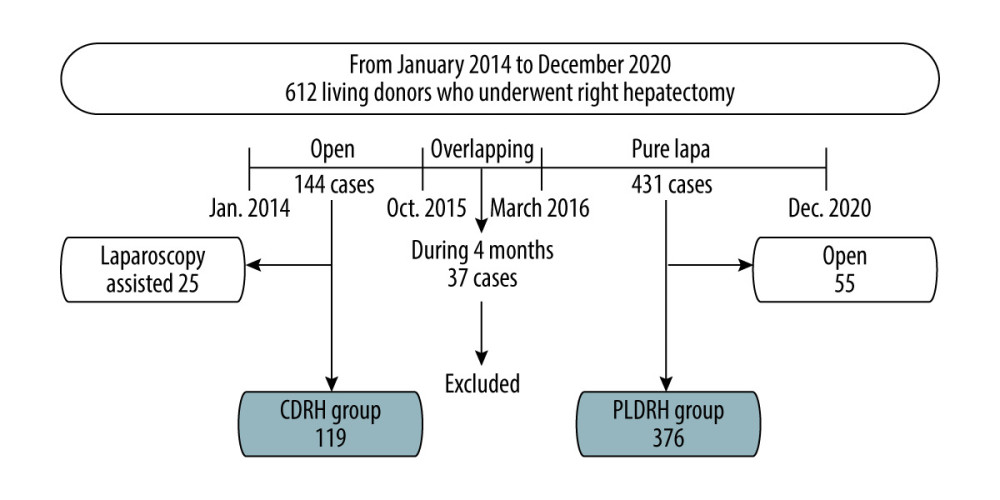
A total of 612 living donors who underwent right hepatectomy between January 2014 and December 2020 at the Seoul National University Hospital (Seoul, South Korea) were enrolled in our study. Donors who underwent extended right hepatectomy were excluded from the study. We divided the patients into 2 groups: the CDRH group, comprising 144 patients who underwent conventional open surgeries between January 2014 and October 2015, and the PLDRH group, comprising 431 patients who underwent PLDH from March 2016 to December 2020. Donors who underwent hepatectomy between these 2 periods were excluded. Donors who underwent laparoscopy-assisted CDRH and CDRH after the standardization of PLDRH were also excluded. Finally, 119 donors from the CDRH group and 376 donors from the PLDRH group were included in the data analysis (Figure 1).
CT VOLUMETRIC PROTOCOL:
All LDLT donors underwent preoperative imaging evaluation with multidetector row helical computed tomography (CT) according to the standard protocol for multiphasic liver CT imaging (reconstruction section thickness, 2.5–3 mm). The EGW was measured using 3D CT image reconstruction software (Dr. Liver; Humanopia Co., Ltd., Pohang-si, South Korea). The surgical team measured the EGW along the planned surgical plane. Gallbladder, blood, and other vascular and biliary structures were excluded during the calculation of liver volume. The density value was set at 1.00 g/mL.
ACTUAL GRAFT WEIGHT MEASUREMENT:
After the liver graft was resected, it was washed out with HTK solution (Custodiol, Kohler Pharma GmbH, Alsbach, Germany) through the main branches at the hilum and cooled to 4°C for preservation. Once the intrahepatic liquid turned clear, the graft weight was measured using a calibrated electronic laboratory scale.
STATISTICAL ANALYSIS:
Results are expressed as mean±standard deviation (SD) for continuous variables and absolute numbers (percentage) for categorical variables. The
Results
We retrospectively reviewed the baseline characteristics and operative outcomes of 495 living donors. The results are summarized in Table 1. The donors in the PLDRH group were slightly younger than those in the CDRH group (36.5±12.7 vs 33.6±10.8 years;
The recipients’ baseline characteristics are summarized in Table 2. The recipients in the PLDRH group were older (55.7±10.9 vs 53.2±12.4 years;
There were no significant differences between the CDRH and PLDRH groups in terms of EGW (792.3±169.9 vs 7r2.2±171.3 g;
Discussion
Since PLDRH was first performed in 2010 [8], it has been performed by experienced surgeons and teams worldwide [9]. Our center previously reported its feasibility and safety based on 300 PLDRH procedures performed from 2016 to 2018 [10]. Accurate graft size is an important factor in liver transplantation that is necessary for donor evaluation and the prevention of small-for-size syndrome (SFSS) in recipients [2]. However, some studies have shown that estimations of graft weight using CT differ from the actual weights [11,12]. Moreover, graft discrepancies in PLDRH have not been fully evaluated.
Our study showed that the EGW was significantly higher than AGW in both the CDRH and PLDRH groups (
Although the EGW was higher than the AGW, there was a significant correlation between the 2. The correlation coefficient (r) was 0.814 in the CDRH group and 0.759 in the PLDRH group, both of which indicate a strong positive correlation. However, the coefficient was slightly higher in CDRH group. Similarly, the R2 value, which shows a linear relationship between the 2 variables, was slightly higher in the CDRH group. The higher discrepancy in the PLDRH group may be due to the use of clips and staplers during PLDRH surgery, whereas the vasculature and bile ducts are incised and sutured during CDRH. The use of clips and staplers takes up space, and thus the division point will be more inclined toward the graft than expected [17]. The division point eventually affects the division plane, which can lead to a decrease in the actual graft weight.
Although there may be other factors that affect the graft weight [3,18,19], there are no large-scale studies investigating these factors and their effects. We analyzed the donor and recipient characteristics to see if there were differences between the PLDRH and CDRH groups, and investigated their influence on the graft weight. Donor age was lower in the PLDRH group. This may be due to PLDRH being preferred for cosmetic purposes and providing a better quality of life [5,10,20]. Thus, the number of young people, especially young women, opting for PLDRH is increasing. Since the donors were young, the number of patients with hypertension seemed to be smaller due to fewer underlying diseases. Kayashima et al [16] suggested that younger donors can develop more capillary vascular beds, which can lead to an increase in the EGW and vulnerability to dehydration. However, the difference was minimal in our study (36.5 vs 33.6 years), and the influence of donor age was reduced in our study by using the HTK solution as the organ perfusion solution, which has a lower osmolarity than the UW (University of Wisconsin) solution that was used in previous studies.
There were more bile duct anomalies in donors in the PLDRH group than there were in the CDRH group. This may be due to the absence of selection criteria for liver anatomy at our center. Although there were few right inferior hepatic vein variations in donors in the PLDRH group, there were still no clear selection criteria. Anatomical variations between the 2 groups were well handled during the surgery. The fact that there was no difference in postoperative complications between the 2 groups demonstrates this.
The underlying etiologies of the recipients were different between the 2 groups. Since the development of direct-acting antiviral agents (DAAs) for hepatitis C virus (HCV), hepatitis C can now be cured and the number of liver transplantations due to HCV has been decreasing [21]. However, with the worldwide increase in alcohol consumption, alcoholic liver disease has increased [22]. Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) have also increased due to the rising prevalence of diabetes and metabolic syndromes [23,24]. As the recipients in the PLDRH group included more recent patients, the number of recipients with HCV-related liver diseases decreased was lower, while other etiologies, including alcoholic liver disease, NAFLD, and NASH, were higher in the PLDRH group. However, recipient etiology did not significantly affect graft weight.
The recipients in the PLDRH group were older and heavier than those in the CDRH group. As population aging continues and surgical techniques develop, recipients’ ages have also increased. In addition, the mean BMI was higher due to changes in eating habits and obesity. Studies have demonstrated that older and heavier people have a poor prognosis in LDLT [25–27]. This may allow operators to cut out more grafts, but the actual graft weight was smaller. In addition, there were only small differences in age or weight between the 2 groups. Therefore, differences in recipient age and weight did not seem to confound the results.
This study also confirmed the general characteristics of PLDRH. The operative time, warm ischemia time, and bench work time were significantly longer, and the blood loss was lower in the PLDRH group, as previously reported [10,28,29]. Laparoscopic procedures are known to have a learning curve [17,30], with the operative time gradually decreasing with the accumulation of the center’s experience. Thus, the mean operation time of PLDRH was 260.9 minutes in this study, which was shorter than that reported in our previous study [10]. Postoperative blood test results were different between the 2 groups. The peak bilirubin and Δbilirubin%, peak AST and ΔAST%, and peak ALT and ΔALT% of the donor were all higher in the PLDRH group than those in the CDRH group. ΔHb% tended to be lower in the PLDRH group (
To the best of our knowledge, this is one of the largest studies to evaluate graft weight discrepancy in patients who underwent PLDRH. However, this study has several limitations. First, the results of this study were from a single center; thus, it may be difficult to generalize our results to other centers. In addition, since this was a retrospective study, there may be a selection bias, such as underreported postoperative complications. Second, there are some factors that may have affected graft weight that have not been fully considered. For instance, Hiroshige et al [15] reported that liver grafts were gradually dehydrated by the organ perfusion solution. Further studies measuring the weight of the liver graft at each step of the back-table procedure are required. Third, the ratio of graft volume (GV) to standard liver volume (SLV), which is another concept used to evaluate graft size for a recipient, was not considered in this study [32,33]. Urata et al demonstrated that the conversion ratio for liver weight and liver volume was 1.12 g/mL, rather than 1.0 g/mL [34]. Furthermore, Toshima et al reported that a recipient’s BMI or physique should be considered (BMI >30 or ≤30 kg/m2) when calculating GV/SLV, to better predict actual SFSS [33]. Considering that our LDLT protocol is based on GRWR with a minimum acceptance of 0.8%, the concept of GV/SLV was not included in the present study. Further studies should include this concept, the actual outcomes according to GV/SLV, and finally, the impact of PLDRH on GV/SLV compared to CDRH.
Conclusions
Graft weight was overestimated in both the PLDRH and CDRH groups. Although the difference between the 2 groups was not substantial, the AGW tended to be smaller in the PLDRH group, which should be taken into account when performing PLDRH.
Tables
Table 1. Baseline characteristics and operative outcomes of liver donors who underwent CDRH and PLDRH.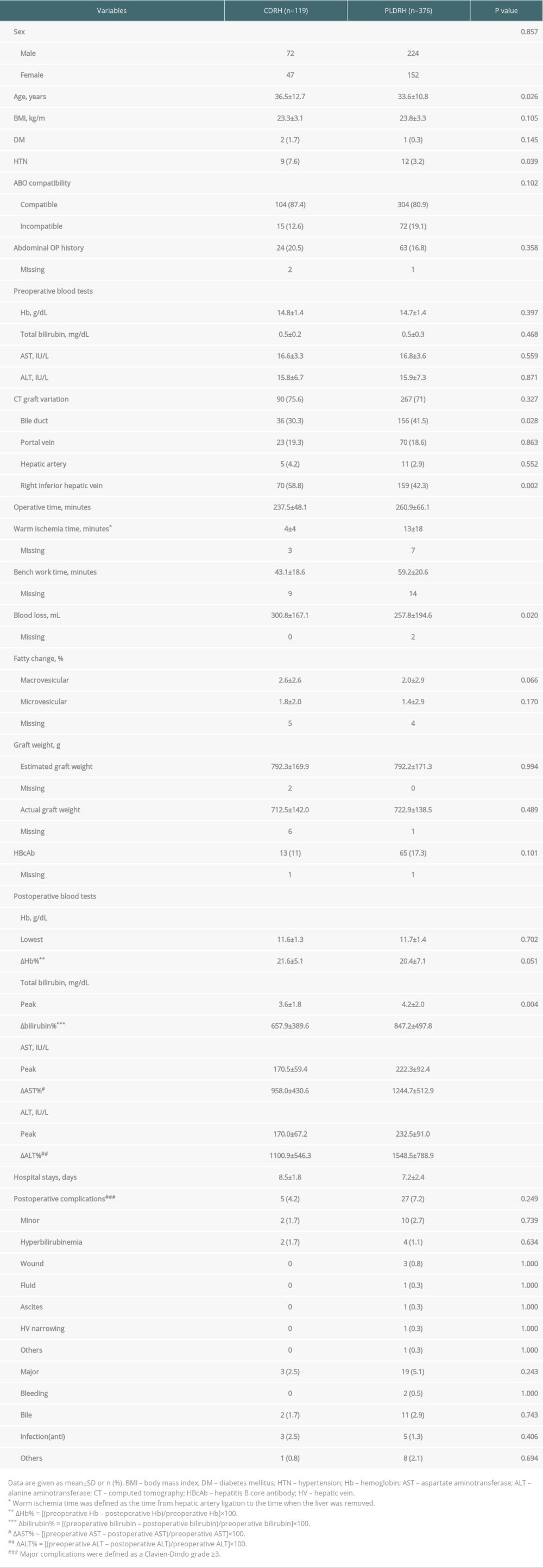 Table 2. Baseline characteristics of CDRH and PLDRH recipients.
Table 2. Baseline characteristics of CDRH and PLDRH recipients.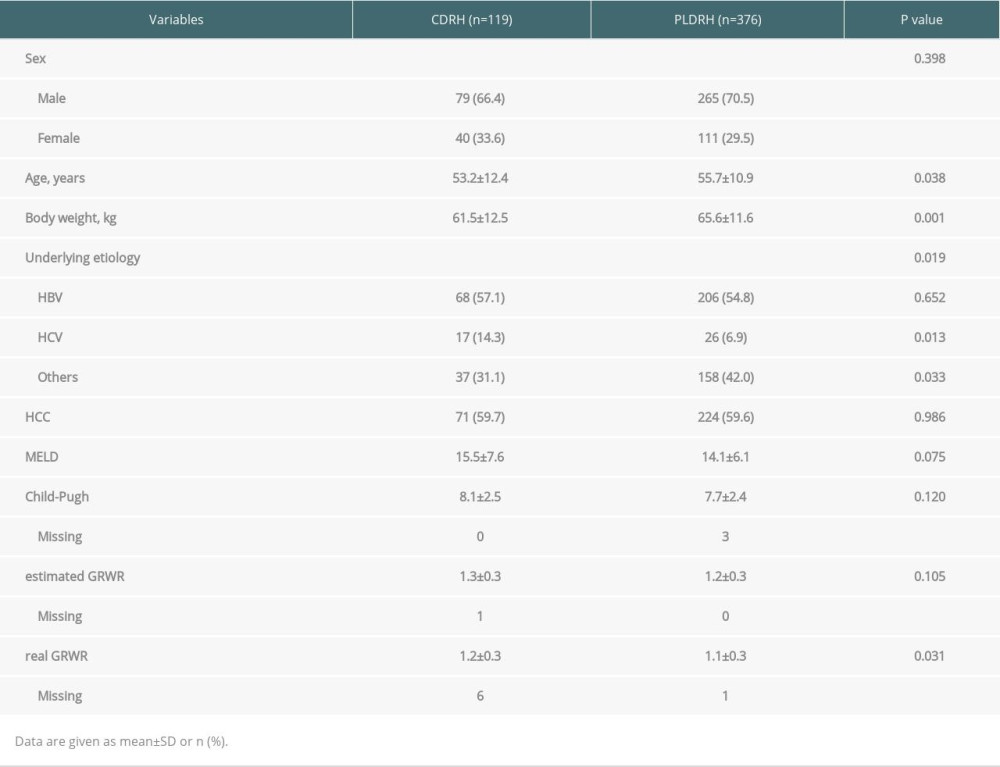 Table 3. Postoperative outcomes of recipients.
Table 3. Postoperative outcomes of recipients.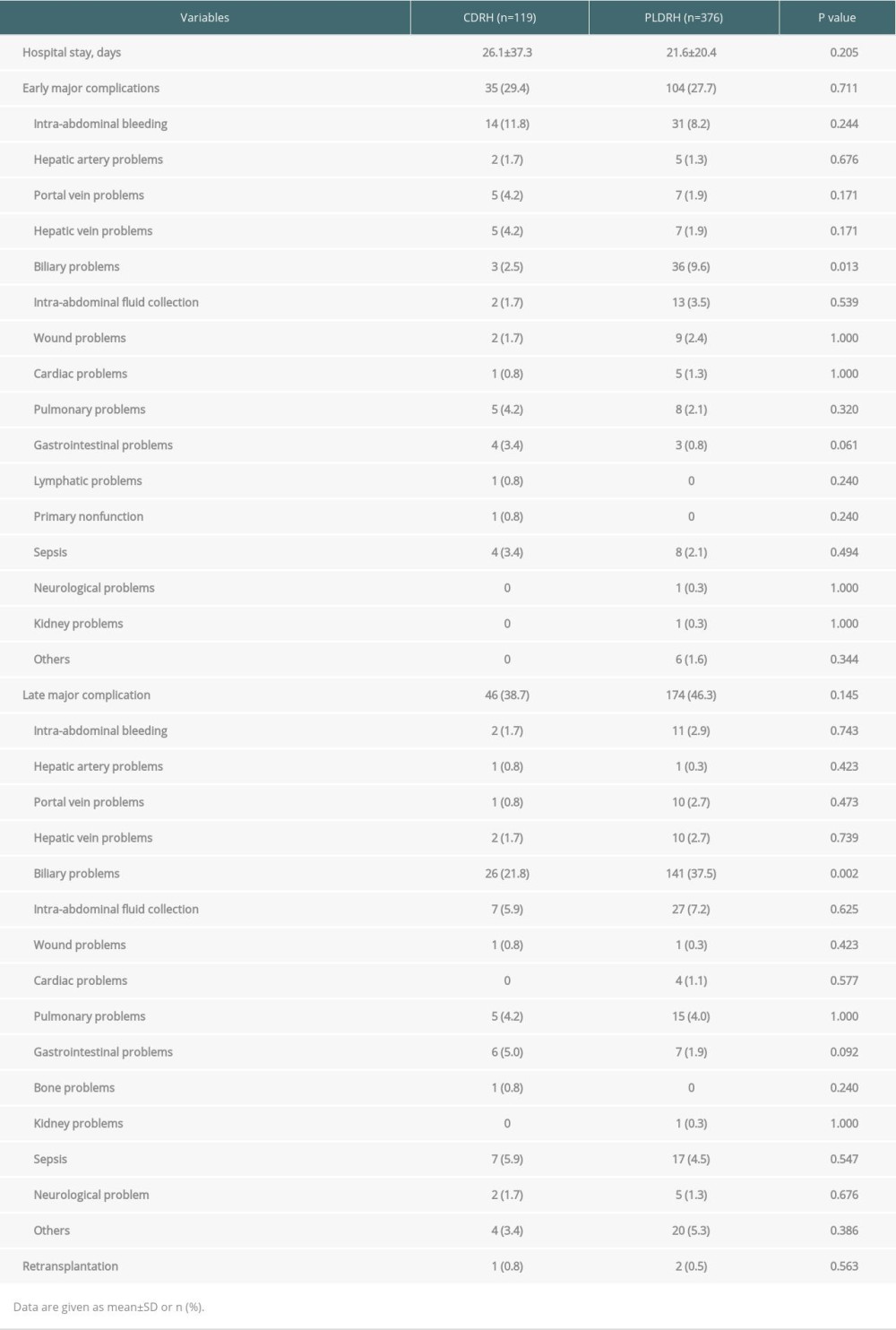 Table 4. Mean estimated graft weights and actual graft weights of CDRH and PLDRH groups.
Table 4. Mean estimated graft weights and actual graft weights of CDRH and PLDRH groups.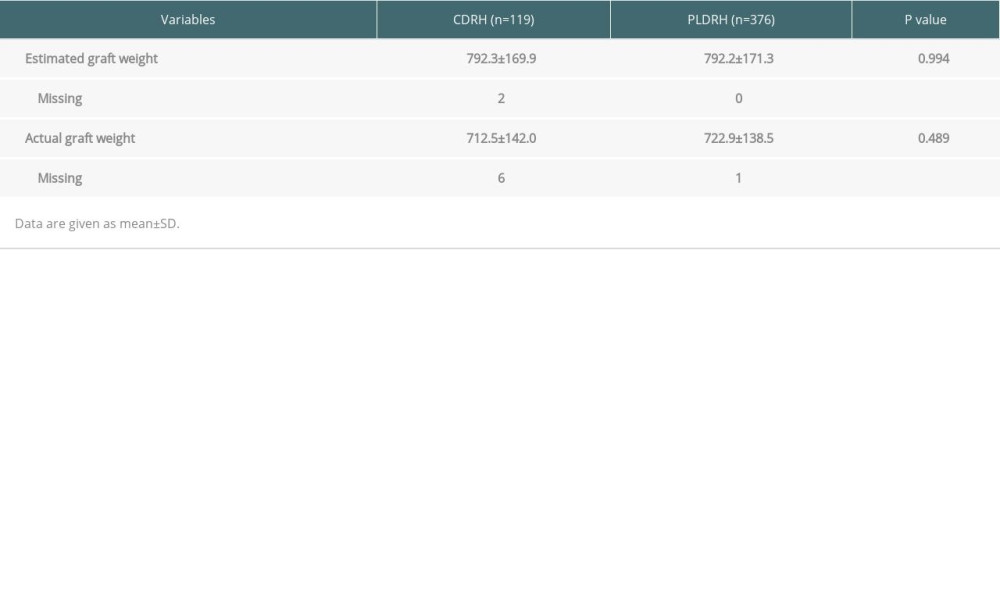
References
1. Chok KS, Fung JY, Chan AC, Comparable short- and long-term outcomes in living donor and deceased donor liver transplantations for patients with Model for End-stage Liver Disease scores ≥35 in a hepatitis-B endemic area: Ann Surg, 2017; 265; 173-77
2. Lemke AJ, Brinkmann MJ, Schott T, Living donor right liver lobes: Preoperative CT volumetric measurement for calculation of intraoperative weight and volume: Radiology, 2006; 240; 736-42
3. Goja S, Yadav SK, Yadav A, Accuracy of preoperative CT liver volumetry in living donor hepatectomy and its clinical implications: Hepatobiliary Surg Nutr, 2018; 7; 167-74
4. Buell JF, Cherqui D, Geller DA, The international position on laparoscopic liver surgery: The Louisville Statement, 2008: Ann Surg, 2009; 250; 825-30
5. Hong SK, Choi GS, Han J, Pure laparoscopic donor hepatectomy: A multicenter experience: Liver Transpl, 2021; 27; 67-76
6. Wakabayashi G, Cherqui D, Geller DA, Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka: Ann Surg, 2015; 261; 619-29
7. Clavien PA, Barkun J, De Oliveira ML, The Clavien-Dindo classification of surgical complications: Five-year experience: Ann Surg, 2009; 250; 187-96
8. Han HS, Cho JY: Total laparoscopic right liver donor hepatectomy, 2012, Seoul, Republic of Korea; Korean Liver Transplant Society
9. Cherqui D, Ciria R, Kwon CHD, Expert consensus guidelines on minimally invasive donor hepatectomy for living donor liver transplantation from innovation to implementation: A joint initiative from the International Laparoscopic Liver Society (ILLS) and the Asian-Pacific Hepato-Pancreato-Biliary Association (A-PHPBA): Ann Surg, 2021; 273; 96-108
10. Hong SK, Tan MY, Worakitti L, Pure laparoscopic versus open right hepatectomy in live liver donors: A propensity score-matched analysis: Ann Surg, 2022; 275; e206-12
11. Pinheiro RS, Cruz RJ, Andraus W, Preoperative computed tomography volumetry and graft weight estimation in adult living donor liver transplantation: Arq Bras Cir Dig, 2017; 30; 38-41
12. Mohapatra N, Gurumoorthy Subramanya Bharathy K, Kumar Sinha P, Three-dimensional volumetric assessment of graft volume in living donor liver transplantation: Does it minimise errors of estimation?: J Clin Exp Hepatol, 2020; 10; 1-8
13. Hwang S, Lee SG, Kim KH, Correlation of blood-free graft weight and volumetric graft volume by an analysis of blood content in living donor liver grafts: Transplant Proc, 2002; 34; 3293-94
14. Radtke A, Sotiropoulos GC, Nadalin S, Preoperative volume prediction in adult live donor liver transplantation: 3-D CT volumetry approach to prevent miscalculations: Eur J Med Res, 2008; 13; 319-26
15. Hiroshige S, Shimada M, Harada N, Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography: Transplantation, 2003; 75; 1561-64
16. Kayashima H, Taketomi A, Yonemura Y, Accuracy of an age-adjusted formula in assessing the graft volume in living donor liver transplantation: Liver Transpl, 2008; 14; 1366-71
17. Suh KS, Hong SK, Lee KW, Pure laparoscopic living donor hepatectomy: Focus on 55 donors undergoing right hepatectomy: Am J Transplant, 2018; 18; 434-43
18. Satou S, Sugawara Y, Tamura S, Discrepancy between estimated and actual weight of partial liver graft from living donors: J Hepatobiliary Pancreat Sci, 2011; 18; 586-91
19. Kitajima T, Kaido T, Tajima T, Younger age is an independent factor for graft weight overestimation: Analysis of the clinical impact on recipient outcomes in 340 Japanese living liver donors: World J Surg, 2018; 42; 218-24
20. Hong SK, Suh KS, Kim KA, Pure laparoscopic versus open left hepatectomy including the middle hepatic vein for living donor liver transplantation: Liver Transpl, 2020; 26; 370-78
21. Fehily SR, Papaluca T, Thompson AJ, Long-term impact of direct-acting antiviral agent therapy in HCV cirrhosis: Critical review: Semin Liver Dis, 2019; 39; 341-53
22. Axley PD, Richardson CT, Singal AK, Epidemiology of alcohol consumption and societal burden of alcoholism and alcoholic liver disease: Clin Liver Dis, 2019; 23; 39-50
23. Friedman SL, Neuschwander-Tetri BA, Rinella M, Mechanisms of NAFLD development and therapeutic strategies: Nat Med, 2018; 24; 908-22
24. Sheka AC, Adeyi O, Thompson J, Nonalcoholic steatohepatitis: A review: JAMA, 2020; 323; 1175-83
25. Durand F, Levitsky J, Cauchy F, Age and liver transplantation: J Hepatol, 2019; 70; 745-58
26. Gil E, Kim JM, Jeon K, Recipient age and mortality after liver transplantation: A population-based cohort study: Transplantation, 2018; 102; 2025-32
27. Diaz-Nieto R, Lykoudis PM, Davidson BR, Recipient body mass index and infectious complications following liver transplantation: HPB (Oxford), 2019; 21; 1032-38
28. Samstein B, Griesemer A, Halazun K, Pure laparoscopic donor hepatectomies: Ready for widespread adoption?: Ann Surg, 2018; 268; 602-9
29. Song JL, Yang J, Wu H, Pure laparoscopic right hepatectomy of living donor is feasible and safe: A preliminary comparative study in China: Surg Endosc, 2018; 32; 4614-23
30. Hong SK, Suh KS, Yoon KC, The learning curve in pure laparoscopic donor right hepatectomy: A cumulative sum analysis: Surg Endosc, 2019; 33; 3741-48
31. Lee KW, Hong SK, Suh KS, One hundred fifteen cases of pure laparoscopic living donor right hepatectomy at a single center: Transplantation, 2018; 102; 1878-84
32. Ikegami T, Onda S, Furukawa K, Small-for-size graft, small-for-size syndrome and inflow modulation in living donor liver transplantation: J Hepatobiliary Pancreat Sci, 2020; 27; 799-809
33. Toshima T, Yoshizumi T, Shimagaki T, Which is better to use “body weight” or “standard liver weight”, for predicting small-for-size graft syndrome after living donor liver transplantation?: Ann Gastroenterol Surg, 2020; 5; 363-72
34. Urata K, Kawasaki S, Matsunami H, Calculation of child and adult standard liver volume for liver transplantation: Hepatology, 1995; 21; 1317-21
Figures
Tables
 Table 1. Baseline characteristics and operative outcomes of liver donors who underwent CDRH and PLDRH.
Table 1. Baseline characteristics and operative outcomes of liver donors who underwent CDRH and PLDRH. Table 2. Baseline characteristics of CDRH and PLDRH recipients.
Table 2. Baseline characteristics of CDRH and PLDRH recipients. Table 3. Postoperative outcomes of recipients.
Table 3. Postoperative outcomes of recipients. Table 4. Mean estimated graft weights and actual graft weights of CDRH and PLDRH groups.
Table 4. Mean estimated graft weights and actual graft weights of CDRH and PLDRH groups. Table 1. Baseline characteristics and operative outcomes of liver donors who underwent CDRH and PLDRH.
Table 1. Baseline characteristics and operative outcomes of liver donors who underwent CDRH and PLDRH. Table 2. Baseline characteristics of CDRH and PLDRH recipients.
Table 2. Baseline characteristics of CDRH and PLDRH recipients. Table 3. Postoperative outcomes of recipients.
Table 3. Postoperative outcomes of recipients. Table 4. Mean estimated graft weights and actual graft weights of CDRH and PLDRH groups.
Table 4. Mean estimated graft weights and actual graft weights of CDRH and PLDRH groups. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860