28 February 2023: Original Paper
Association Between Dietary Quality and Serum Cystatin C in Kidney Transplant Recipients Based on Chinese Diet Balance Index 2016 (DBI-16)
Hailing Zhang12AE, Ke Shi3AE, Li Yuan14CD, Xiaohong Guan15BC, Haihui Yin16D, Wenjing Zhao3F, Xinyi Zhou1B, Aiqin Chu1A*DOI: 10.12659/AOT.939149
Ann Transplant 2023; 28:e939149
Abstract
BACKGROUND: Cystatin C (Cys) is considered to be a better marker than serum creatinine in assessing kidney function, predicting cardiovascular events, and all-cause mortality. It seems to be associated with nutritional status in the general population, but little is known about kidney transplant recipients (KTRs). This study aimed to explore the relationship between dietary balance index and serum Cys in KTRs.
MATERIAL AND METHODS: In a cross-sectional study, 215 KTRs completed an FFQ questionnaire and information on serum Cys. Dietary intake was assessed using the Food Frequency Questionnaire (FFQ). Dietary Balance Index 2016 (DBI-16) edition scores were calculated as an indicator of dietary quality. Data on the patient’s serum Cys were obtained through the hospital information system.
RESULTS: The majority of KTRs were male (75.34%), 76.74% were aged 18-44 years, and 79.53% were abnormal serum Cys. Dairy (z=-2.161, P<0.05), meat (z=-2.578, P<0.05), and dietary diversity (z=-3.393, P<0.05) in the normal group were higher than those in the abnormal group, and the dietary quality distance (DQD) score (t=-2.264, P<0.05) was lower than that in the abnormal group. After adjusting for confounders, a low-quality diet was a risk factor for maintaining the normal level of serum Cys (OR 3.022, 95% CI 1.263-7.231, P<0.05).
CONCLUSIONS: The present study suggested that KTRs with a high dietary quality might be associated with normal serum Cys levels. Dairy, meat, and varied diet seems to impact the serum Cys levels of KTRs. Dietary imbalances were prevalent among KTRs.
Keywords: CST3 Protein, Human, Diet Therapy, Kidney Transplantation, Female, Humans, Male, Cross-Sectional Studies, cystatin C, Diet, East Asian People, transplant recipients, Adolescent, young adult, Adult
Background
In recent years, the number of patients with end-stage kidney disease in China has increased each year [1,2]. The long-term survival rate of those who have received a kidney transplant is much higher than for those who undergo dialysis treatment; therefore, a kidney transplant has become the first-choice treatment for those with end-stage kidney disease [3,4]. According to the World Health Organization Global Donor and Transplantation Database, 65,116 kidney transplants were performed worldwide in 2017 [5]. The Chinese Kidney Transplant Scientific Registry showed that China ranked second in the number of kidney transplants performed in 2020, at more than 150,000 [6]. Although the 1-year survival rate for those who have received a transplanted kidney is as high as 95% after existing surgical, immunosuppression, and monitoring protocols are followed, the long-term survival of kidney transplant recipients (KTRs) has not significantly improved [7]. Studies have shown that cardiovascular disease, rejection, diabetes, infection, and other complications after transplantation seriously affect the long-term prognosis and quality of life of KTRs [8].
Serum cystatin C(Cys) is considered to be a better marker than serum creatinine in assessing kidney function, predicting cardiovascular events, and all-cause mortality [9–12]. In addition, serum Cys is a reliable biochemical indicator for detecting kidney function and identifying risk factors for postoperative transplantation kidney function in KTRs [13–15]. Studies have showed that a serum Cys -based equation is a better predictor of allograft function than a creatinine-based equation and is a good proxy for monitoring allograft function [16]. Hošková et al showed that the level of serum Cys can predict acute kidney injury after transplantation [17]. Serum Cys is not only affected by age, sex, race, and smoking, but is also likely affected by diet [15]. Magnusson et al confirmed that serum Cys seems to be related to metabolic syndrome, but metabolic syndrome is easily affected by dietary factors [18]. Vallianou et al found that the increase in Mediterranean diet score in healthy people is related to the decrease of serum Cys level, which means that the Mediterranean diet can reduce serum Cys levels, thus reducing vascular risk [19]. Ludwig-Borycz et al found that in the elderly, consumption of milk, eggs, and meat is related to the level of serum Cys, and organic food consumption can reduce the level of serum Cys [20]. What’s more, studies have shown effective nutritional management is the best strategy for prolonging graft survival, improving graft kidney function, and reducing all-cause mortality in KTRs [21]. However, little is known about the relationship between serum Cys and diet in KTRs.
Recent research on nutrition in KTRs has focused on the intake of nutrients or foods and how this relates to control of complications [22–24]. Two US longitudinal studies have shown that diet quality seems to be associated with serum Cys in the elderly, with high-quality diets leading to healthier serum cystatin C outcomes, but less research has been conducted on the relationship between diet quality and serum Cys in KTRs [25,26]. Studies have shown that due to the complexity of diets, dietary quality is a more reliable nutritional monitoring indicator than nutrition [27]. It is very important to use reliable indicators of dietary quality to evaluate the relationship between diet and disease. In recent years, various dietary evaluation indicators have been developed around the world. Examples are the desirable dietary pattern (DDP), the healthy eating index (HEI), and the dietary quality index (DQI), which are based on Western dietary patterns. The diet balance index-16 (DBI-16) is based on the dietary guidelines and the balanced diet pagoda for Chinese residents. Its advantage is that it can evaluate dietary quality as a whole and can also evaluate the deficiency and excess of dietary intake, and it has been widely used to evaluate dietary quality of all kinds of people in China [28]. Thus, it provides better guidance for the target population in preventing the occurrence of nutrition-related diseases. In the present study, we aimed to assess the dietary quality of KTRs using the DBI-16, and to explore the relationship between dietary quality and serum Cys in KTRs.
Material and Methods
PARTICIPANTS:
Through the convenience sampling method, patients who were revisited in the kidney transplant outpatient clinic of the First Affiliated Hospital of University of science and technology of China were selected as subjects from October 2020 to April 2021. Based on Kendall’s proposal that the sample size of studies on variables influencing factors is at least 5 to 10 times the number of variables, a total of 21 variables in this study, calculated according to the sample size is 10 times the number of variables, this study needed at least 210 patients. There were 228 KTRs who volunteered to participate in the study, and those with incomplete information were excluded, resulting in 215 participants. The participants were recipients of single-kidney transplants. Exclusion criteria were: (1) recipients of multi-organ transplants, (2) patients with language disorders, (3) patients with cognitive impairment, (4) daily energy intake exceeded the usual permissible ranges of 500 to 3500 kcal/d for women and 800 to 4000 kcal/day for men.
STUDY DESIGN:
This was a cross-sectional study. Prior to the formal survey, 2 investigators were uniformly trained by a faculty member specializing in nutrition. After patients presented themselves at the KTR clinic, the researchers collected their demographic information and dietary data using a questionnaire. These patients’ medical records were obtained through the hospital information system. The participating patients were taken to a quiet consultation room where the researchers interviewed the patients face-to-face using a questionnaire that was completed on the spot by the researcher. To accurately assess the patient’s intake of various foods, the researcher showed the patient the mold of a food container as a reference. The interview took approximately 30 minutes per patient, and it took approximately 10 minutes to extract the laboratory test reports related to kidney function. The 2 researchers then entered the data from the completed questionnaire and the laboratory data into an Excel spreadsheet. The data were then handed over to the data analysis team. The data analysis team did not know the patients’ identifying information and thus could not relate the data to individual patients.
DEMOGRAPHIC DATA:
Demographic information included age, sex, height, place of residence, education, weight, height, history of smoking and alcohol consumption, source of the transplanted kidney, time since transplantation, mode of dialysis prior to kidney transplantation, and drug treatment plan. We calculated a volume body mass index (BMI) and, in accordance with the reference standard for the Chinese population, patients were divided into an underweight group (BMI <18.5), a normal weight group (18.5≤ BMI <24), and an overweight group (BMI ≥24). We classified age according to the World Health Organization’s criteria for age groups as a youth (18–44 years) and middle-aged (45–59 years). The drug treatment plan included a tacrolimus treatment plan and a cyclosporine treatment plan.
DIETARY INTAKE INFORMATION: The Food Frequency Questionnaire (FFQ) [29], which has been validated for reliability, was used in combination with food molds to collect information on the patient’s dietary intake in the past year by means of face-to-face interviews. The FFQ questionnaire was based on foods with high intake frequency as determined by the 2002 China National Nutrition Survey and adjusted for the dietary habits of the local population. A total of 131 different kinds of food were included. We classified these foods into 9 categories: cereals, vegetables, fruits, dairy products, legumes, animal foods, aquatic foods, eggs, and pure energy foods. We also added the categories of edible oils and spices. Oils and spices were assessed based on the patient’s monthly consumption list.
:
The DBI-16 is an effort to evaluate dietary quality more rationally under the guidance of the Dietary Guidelines for Chinese Residents published in 2016. It contains 8 food indicators, namely (range of values) (1) Cereals (−12–12) (2) Fruits and vegetables (vegetables 6-0, fruits 6-0); (3) Dairy and soybeans (dairy 6-0, soybeans 6-0); (4) Animal foods (meat 4–4, fish 4-0, eggs 4–4); (5) Pure energy foods (cooking oil 0–6, alcohol 0–6); (6) Condiments (sugar 0–6, salt (0–6); (7) Varied diet (−12–0); and (8) water (−12–0). If data on water consumption are lacking, they could be ignored in the evaluation. A varied diet was calculated based on 12 food groupings: rice, pasta, coarse grains and potatoes, dark vegetables (≥500 μg carotenoids per 100 g of vegetables), light vegetables (<500 μg carotenoids per 100 g of vegetables), fruits, soy products, dairy products, livestock, poultry, eggs, and fish and shrimp.
The low bound score (LBS) of the scale is the sum of the absolute values of all negatively assigned index scores, reflecting inadequate dietary intake, and ranges from 0 to 60. The high bound score (HBS) of the scale is the sum of all positively assigned index scores, reflecting excessive dietary intake, and ranges from 0 to 38. The dietary quality distance (DQD) is the sum of the absolute values of each index, reflecting dietary imbalance. A score of 0 indicates good dietary intake (Suitable), a score that is below 20% of the total score indicates good dietary intake (More suitable), and a score that is 20–40% of the total score indicates acceptable dietary intake (Low level), a score that is 40–60% of the total score indicates poor dietary intake (Medium level), and a score that is more than 60% of the total score indicates the worst dietary intake (High level), also defined as poor dietary quality [30]. Data on water and sugar were lacking, so scores for added sugar and drinking water were not accounted for [31].
SERUM CYS: We extracted data on the serum Cys of the KTRs from the hospital information system. These medical reports were obtained from the Department of Laboratory Medicine. Serum Cys was detected using an immunoturbidimetric method in a Siemens ADVIA 2400 instrument. The normal range for serum Cys is 0.59 to 1.03 mg/L; this range was obtained from the fourth edition of the China National operational protocol for clinical testing [32].
STATISTICAL METHODS:
Data were analyzed using SPSS 26.0. The χ2 test was used to detect differences in rates of general demographic information (eg, age, sex, BMI). The Shapiro-Wilk U test was used to assess normality (eg, DBI scores). Mann-Whitney U test is used to detect the differences between groups with abnormal distribution (eg, cereals, vegetables, fruits), and the t test was used to compare the differences between groups with a normal distribution (eg, DQD score). Subject work characteristic curves (ROC) were constructed to obtain DBI-16 dietary quality distance score cut-offs, and patients were divided into relatively low and high dietary quality groups [33]. Variables to be adjusted were selected based on clinical relevance or significance (P<0.05) in the univariate analysis. Logistic regression models were constructed to examine the relationship between overall dietary quality and serum Cys.
Results
CHARACTERISTICS OF THE PARTICIPANTS:
A total of 215 KTRs were investigated. They consisted of 162 (75.34%) males and 53 (24.65%) females; 165 (76.74%) of whom were aged 18 to 44 and 70 (32.56%) aged 45 to 59; 26 (12.10%) had a BMI of <18.5 kg/m2, 129 (60.00%) had a BMI of ≤18.5 kg/m2, and 60 (27.91%) had a BMI of ≥24 kg/m2; 157 (73.02%) had never been smokers, 47 (21.86%) were former smokers, and 11 (5.12%) were current smokers; 117 (54.42%) engaged in physical activity and 98 (54.58%) did not engage in physical activity. Of the KTRs, 181 (84.19%) had received their kidney from a related living donor and 34 (15.81%) from a cadaveric donor; 98 patients (45.58%) had undergone their kidney transplant surgery less than 1 year ago, 65 (30.23%) between 1 year and less than 3 years ago, and 52 (24.19%) 3 or more years ago. There were 152 (70.70%) hemodialysis patients, 43 (20.00%) peritoneal dialysis patients, and 20 (9.30%) non-dialysis patients before the transplant operation. Of the KTRs, 155 (72.09%) took tacrolimus orally and 60 (27.91%) took cyclosporine orally. Sex (χ2=12.89, P<0.001) and drugs (χ2=12.229, P<0.001) had an effect on serum Cys. Age, BMI, smoking, physical activity, source of the transplanted kidney, and preoperative dialysis mode had no effect on serum Cys (P>0.05) (Table 1).
DIETARY INTAKE AND DIETARY QUALITY SITUATION:
Table 2 shows the scores for the DBI-16 components and the percentage of participants with each score. Among patients with normal serum Cys, there was an excessive intake of cereals (97.72%), meat (68.18%), eggs (54.55%), and oil (54.54%), and an insufficient intake of vegetables (54.54%), fruits (47.73%), dairy products (90.91%), beans (84.09%), and fish (63.64%), as well as an insufficiently varied diet (93.18%). Among patients with abnormal serum Cys, there was an excessive intake of cereals (98.81%), meat (52.04%), eggs (39.19%), and oil (49.71%), and an insufficient intake of vegetables (43.85%), fruits (56.14%), dairy products (93.57%), beans (73.68%), and fish (70.76%), and also an insufficiently varied diet (97.66%).
Table 3 shows the distribution of the DBI-16 indicators among the participants. An LBS score indicated insufficient food intake, with 6.82% of patients with serum Cys having moderate or high food intake deficiency, in comparison to 14.62% of patients with abnormal serum Cys. An HBS score was an indication of excessive food intake, with 54.55% of patients with normal serum Cys having a higher food intake level, in comparison to 44.44% of patients with abnormal serum Cys. DQD is an index that is used to evaluate the overall imbalance with regard to dietary intake. Of patients with normal serum Cys, 38.64% had a moderate or high food intake imbalance, and 49.7% of patients with abnormal serum Cys had a moderate or high food intake imbalance.
COMPARISON OF DBI-16 SCORES WITH COMPONENTS BETWEEN THE 2 GROUPS:
Table 4 shows the DBI-16 scores for each group. Dairy (z=−2.161, P<0.05), meat (z=−2.578, P<0.05), and dietary diversity (z=−3.393, P<0.05) in the normal serum Cys group were higher than those in the abnormal serum Cys group, and the dietary quality distance (DQD) score (t=−2.264, P<0.05) was lower than that in the abnormal group. Cereals, fruits, vegetables, beans, fish, eggs, oil, and salt had no significant effect on the value of cystatin C (P>0.05).
BINARY LOGISTIC REGRESSION ANALYSIS OF THE POTENTIAL ASSOCIATION BETWEEN DIETARY QUALITY AND SERUM CYS:
According to the DBI-16 scale, less than 8% of patients had a high-quality diet, and it was not possible to group patients according to the quality of their diet (Table 3). Table 5 show that the area under the ROC curve was 0.604, the sensitivity was 39.1%, and the specificity was 81.8% (P=0.034). We used 45.5% of the DBI-16 dietary quality score (35.5 points) to classify kidney transplant recipients into a lower-quality diet group and a higher-quality diet group. Those with a DBI-16 dietary quality score of >35.5 were in the lower-quality diet group, and those with a score of 35.5 or lower were in the higher-quality diet group.
Table 6 shows that using serum Cys as the dependent variable and adjusting for sex and drugs, patients with a lower-quality diet were 3.071 times more likely to have abnormal serum Cys than those with a higher-quality diet (OR 3. 022, 95% CI 1. 263–7. 231, P=0.013).
CORRELATION ANALYSIS BETWEEN COMPONENTS OF DBI-16 AND EGFR BASED ON SERUM CYSTATIN C:
Table 7 shows that in the DBI-16 grouping score, meat (r=0.141, P<0.05), varied diet (r=0.179, P<0.001), and DQD (r=0.168, P<0.05) were related to eGFR based on cystatin C.
Discussion
This was a cross-sectional study to evaluate the association of dietary quality with serum Cys in 215 kidney transplant recipients attending the kidney transplant outpatient clinic of a hospital in China. Of the whole sample, we observed that KTRs with a low-quality diet were 3.022 times more likely to have abnormal serum Cys than KTRs with a high-quality diet. The DQD, dairy, meat, and varied diet scores of the 2 groups differed significantly. Dietary imbalances are common in KTRs. It is likely that dietary quality is a limited risk predictor of serum Cys abnormalities. Dairy, meat, and varied diet scores may be protective of serum Cys.
Kidney transplantation as the preferred form of kidney replacement therapy is both cost-effective and has survival advantages, extending life expectancy by 7 to 11 years, depending on the cause of kidney failure [34]. Dyslipidemia is a common complication of kidney transplantation and can increase the risk of cardiovascular complications, which are the leading cause of death in the transplant population [35]. Serum Cys has been identified as a sensitive indicator of kidney dysfunction associated with cardiovascular events [15]. Preventive measures include regular physical activity and a normal calorie diet with an emphasis on vegetables, fruits, whole grains, legumes, healthy sources of protein, and non-tropical vegetable oils [31]. Our study found that KTRs with normal serum Cys had a higher intake of fruits and milk than KTRs with abnormal cystatin and that the difference was statistically significant. This finding is in line with that of the Wai et al study, which found that an increase in fruit intake could delay kidney failure [35], and with that of the Silva et al study, which found that dairy products were beneficial for kidney excretion [36]. Qingqing Cai et al suggested that high meat intake is a risk factor for the functioning of a transplanted kidney [23]. The association of dairy products with cystatin C appears to be due in part to the potentially protective components of dairy products, including monounsaturated fat, polyunsaturated fat, phosphorus, magnesium, calcium, and vitamin D [37]. Second, it is possible that the fat content of some dairy products counteracts the beneficial effects of other nutrients in dairy products. Further observational and experimental work is needed to explore the underlying mechanisms behind this association. However, our study found that the average meat intake was higher in kidney transplant recipients with normal serum Cys than in KTRs with abnormal serum Cys. There may be 3 reasons for this. First, the overall meat intake of the population that we investigated was not excessive. Second, the diet of the population that we investigated was based mostly on cereals and vegetables, in contrast to the meat-based dietary pattern observed in the above study. Finally, the people we investigated generally did not eat a lot of beans, dairy products, or fish, and their main source of protein was red meat. However, inadequate protein intake, increased nutritional losses, inflammation, oxidative stress, and anabolic or catabolic disorders have been found to lead to high glomerular filtration rates and abnormal kidney function [38–40]. The association of meat foods and cystatin C may be attributed to changes in circadian rhythms or hormonal effects of diet. Toffaletti et al showed that cystatin C appeared to decrease slightly after beef ingestion by some, in agreement with the conclusion of our study [41]. There is evidence that inflammation, thyroid hormone levels, and immunosuppressive drugs (eg, steroids) affect plasma cystatin C concentrations, which have been reported to decrease in patients with hyperthyroidism when treated with antithyroid drugs, thyroid surgery, or radioiodine [42].
Dietary intake and dietary quality tests are more informative about the overall diet and clinical outcomes of KTRs [43]. Our study showed that dietary diversity scores were, on average, higher in the serum Cys normal group than in the serum Cys abnormal group. A study found that dietary diversity improved the nutritional status of KTRs, helped them to control their weight, and thus influenced their clinical outcome [44]. Therefore, we speculate that dietary diversity is associated with serum Cys, possibly due to the diverse diet being able to meet the patient’s diverse nutritional requirements, contributing to a healthier weight and better clinical outcomes. In addition, we found that the dietary quality of the serum Cys normal group was higher than that of the serum Cys abnormal group. The lack of research on the overall quality of diets of KTRs makes direct comparisons with other studies difficult, but Zhong et al suggested that a high-quality diet is associated with a lower absolute long-term risk of cardiovascular disease and mortality and a longer period of cardiovascular disease-free survival [45]. Cardiovascular disease is a common complication that affects kidney function after kidney transplantation. The main risk factors for cardiovascular disease are dyslipidemia, weight gain, and increased blood glucose, which appear to be related to diet [46]. We calculated the eGFR based on the values of serum cystatin C and did a Pearson correlation analysis between the eGFR and the values of the DBI-16 components. We found that DQD scores were negatively correlated with eGFR, but a higher DQD score indicates lower diet quality. The meat and varied diet scores were positively correlated with eGFR. Therefore, we speculate that higher dietary quality may be associated with lower risk factors of cardiovascular disease influencing the function of a transplanted kidney, which in turn correlates with the levels of cystatin C.
The innovation in this study is the use of DBI-16 as an indicator of dietary quality because DBI-16 can overcome the limitations of traditional methods of assessing dietary quality. The limitations of this study are that this was a cross-sectional survey of a small sample, and the use of a retrospective dietary survey method may have resulted in errors due to the poor memory of patients. There is currently a dearth of experimental studies on dietary quality and serum Cys in relation to KTRs. Therefore, we cannot rule out biases in the correlation between dietary quality and the risk of abnormal levels of serum Cys. We suggest that in the future, investigators should focus on the impact of dietary quality on kidney function and conduct experimental studies to further explore the impact of dietary quality on serum Cys levels in KTRs. In addition to this, the present study served as a cross-sectional analysis, confounding factors are difficult to remove, and observational studies are needed with 1-year or longer follow-up.
Conclusions
We found that KTRs with a high dietary quality tend to have normal serum Cys levels. Consuming dairy products and meat and having a varied diet seems to impact the serum Cys levels of KTRs. Dietary imbalances were prevalent among KTRs.
Tables
Table 1. Characteristics of the study participants as stratified by the serum cystatin C index.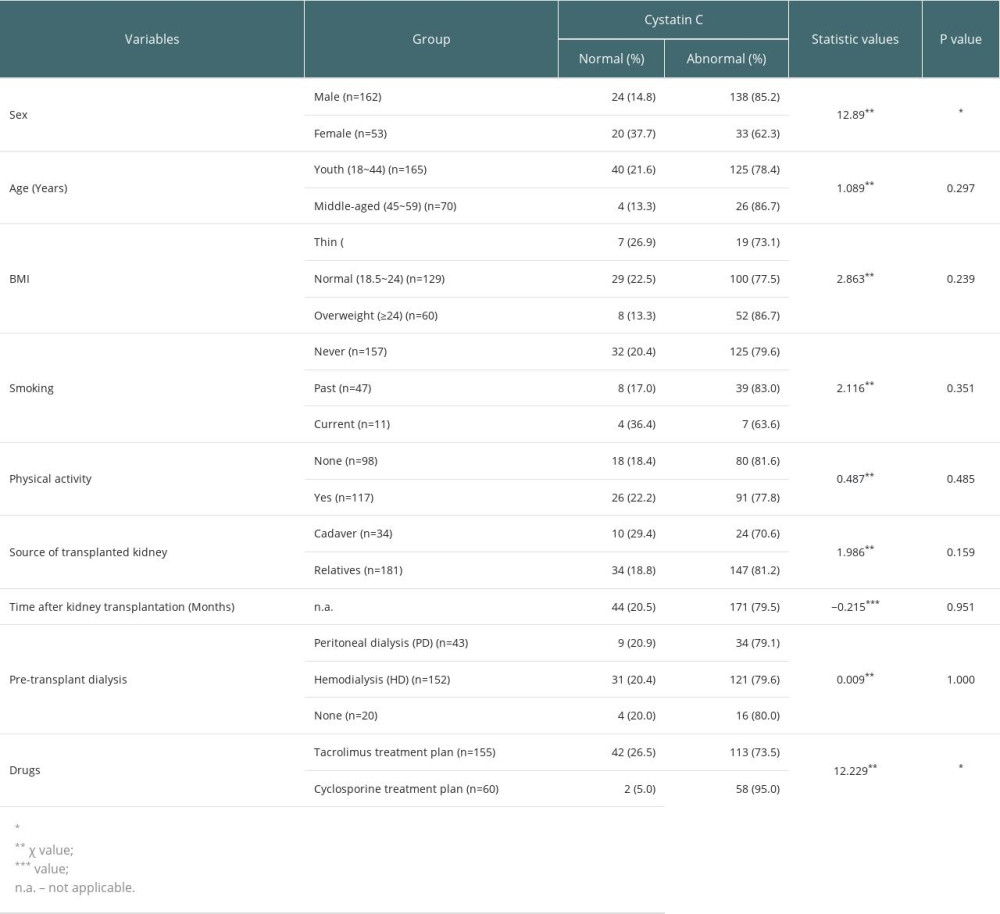 Table 2. Scores for the DBI-16 components and the percentages of participants with each score in the 2 groups.
Table 2. Scores for the DBI-16 components and the percentages of participants with each score in the 2 groups.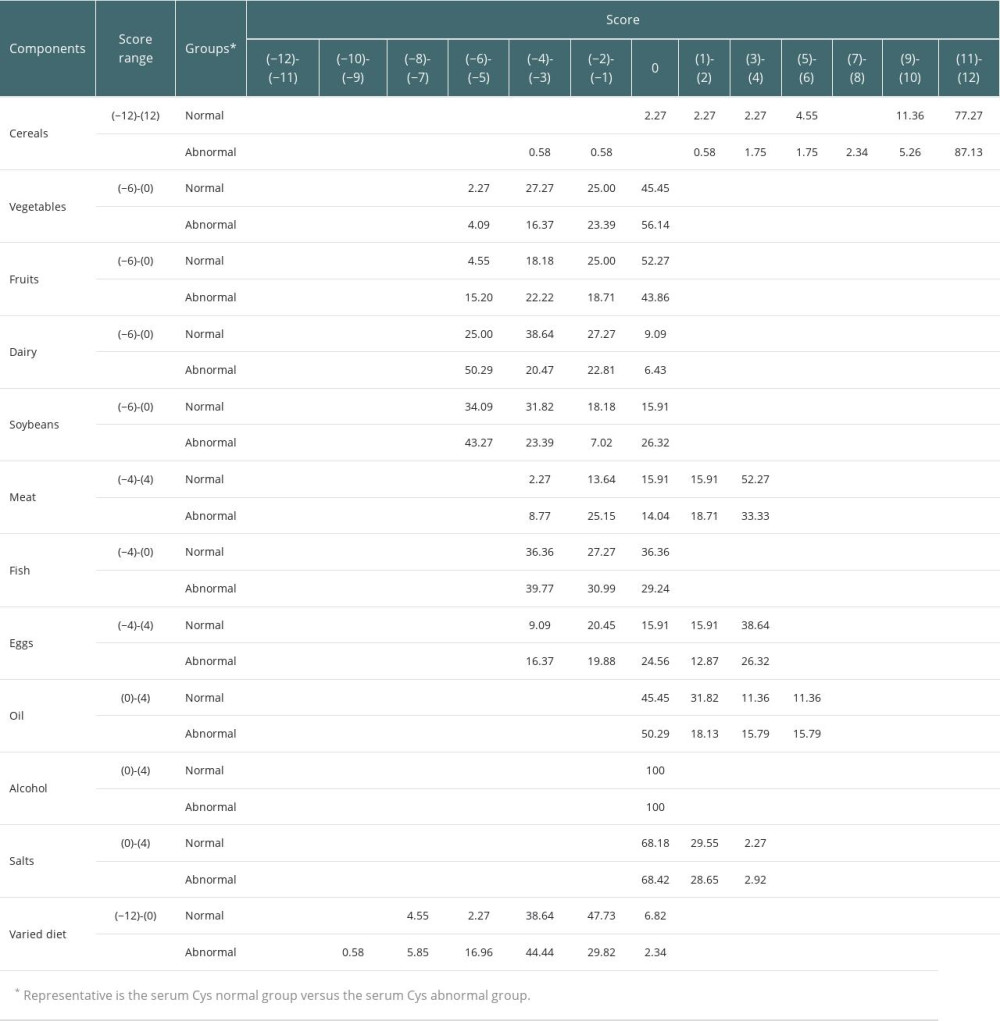 Table 3. Distribution of DBI-16 indicators among the participants.
Table 3. Distribution of DBI-16 indicators among the participants.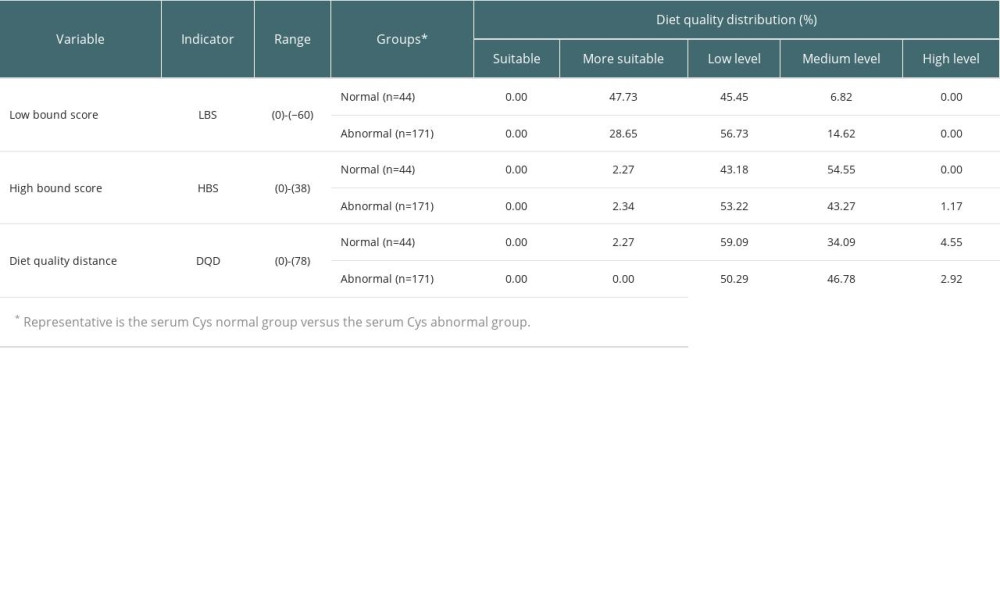 Table 4. A comparison of component DBI-16 scores between the groups.
Table 4. A comparison of component DBI-16 scores between the groups.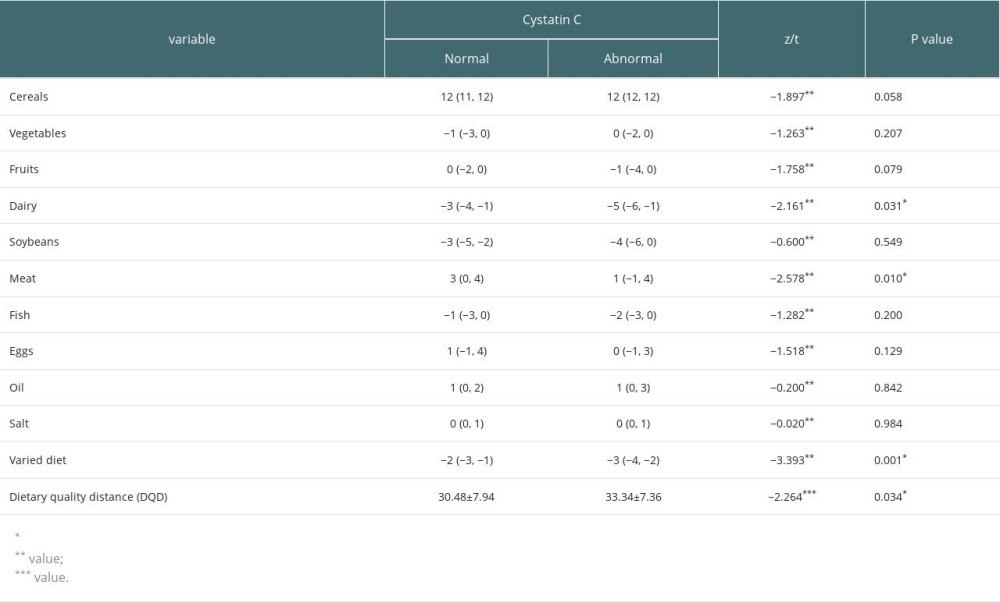 Table 5. Subject Work Characteristics (ROC) curve results.
Table 5. Subject Work Characteristics (ROC) curve results.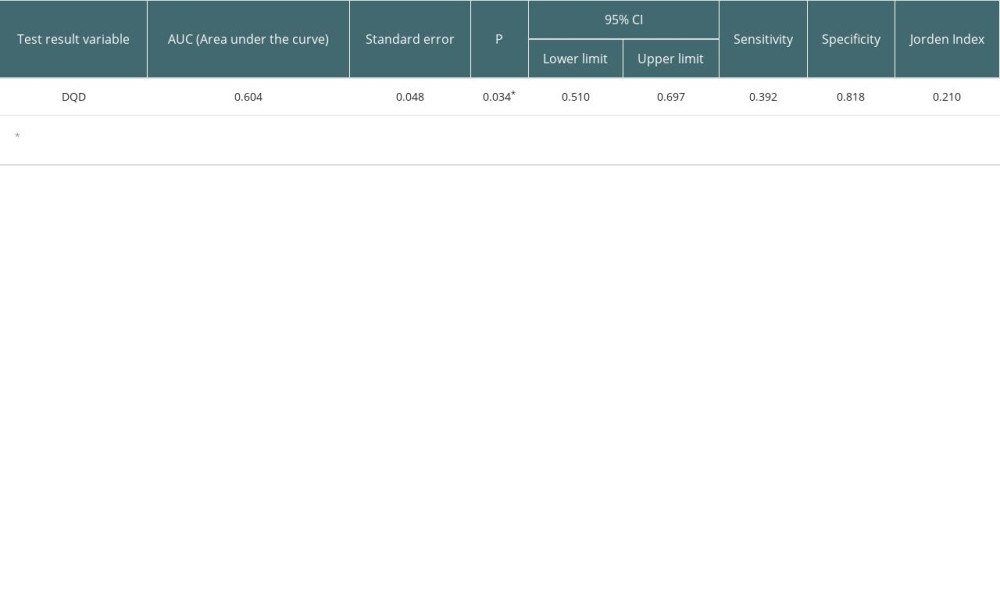 Table 6. A logistic regression model of dietary quality and cystatin C in renal transplant recipients.
Table 6. A logistic regression model of dietary quality and cystatin C in renal transplant recipients.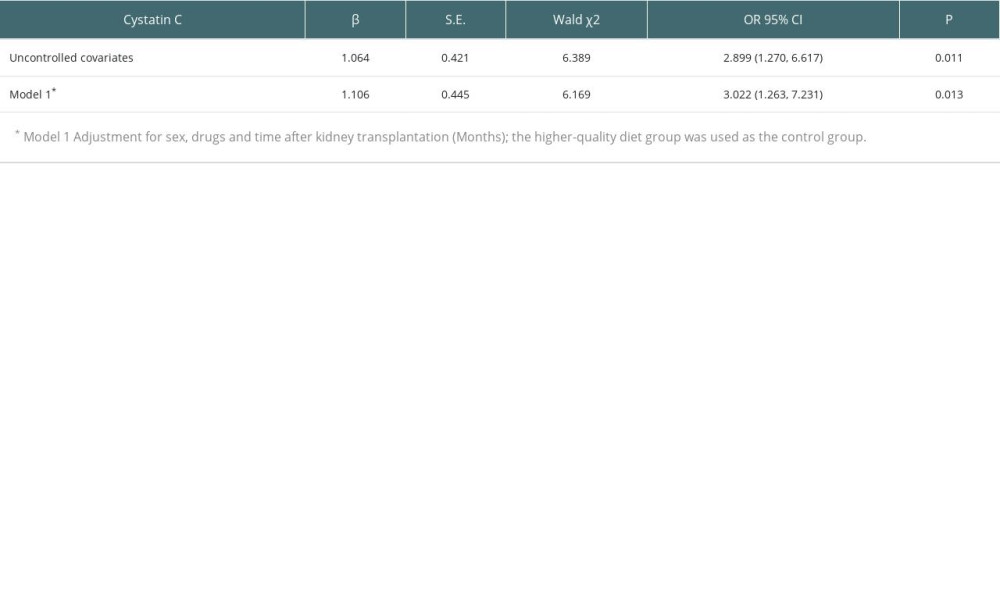 Table 7. Correlation analysis between components of DBI-16 and eGFR based on serum cystatin C(r).
Table 7. Correlation analysis between components of DBI-16 and eGFR based on serum cystatin C(r).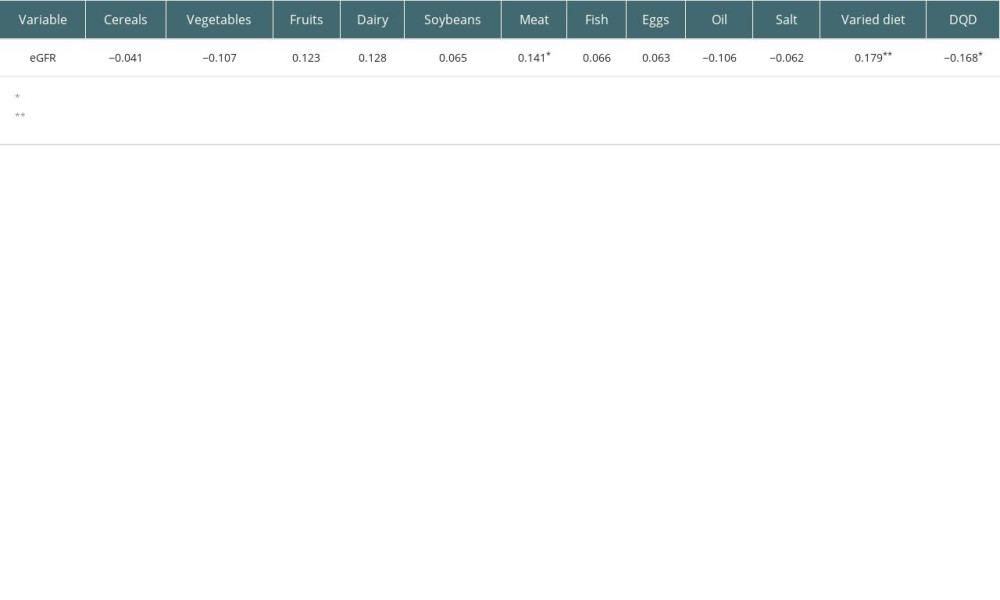
References
1. GBD Chronic Kidney Disease Collaboration, Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study: Lancet, 2020; 395; 709-33
2. Zhang L, Wang F, Wang L, Prevalence of chronic kidney disease in China: A cross-sectional survey: Lancet, 2012; 379; 815-22
3. Goldfarb DA, Renal transplantation and renovascular hypertension: J Urol, 2021; 205; 1214-15
4. Jamieson NJ, Hanson CS, Josephson MA, Motivations, challenges, and attitudes to self-management in kidney transplant recipients: A systematic review of qualitative studies: Am J Kidney Dis, 2016; 67; 461-78
5. Loupy A, Aubert O, Orandi BJ, Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study: BMJ, 2019; 366; l4923
6. Caballero B, Humans against obesity: Who will win?: Adv Nutr, 2019; 10; 4-9
7. Viklicky O, Novotny M, Hruba P, Future developments in kidney transplantation: Curr Opin Organ Transplant, 2020; 25; 92-98
8. Ojo AO, Cardiovascular complications after renal transplantation and their prevention: Transplantation, 2006; 82; 603-11
9. Dharnidharka VR, Kwon C, Stevens G, Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis: Am J Kidney Dis, 2002; 40; 221-26
10. Shlipak MG, Katz R, Sarnak MJ, Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease: Ann Intern Med, 2006; 145; 237-46
11. Foley RN, Wang C, Collins AJ, Cystatin C, mortality risk and clinical triage in US adults: threshold values and hierarchical importance: Nephrol Dial Transplant, 2011; 26; 1831-37
12. Allen AM, Kim WR, Larson JJ, Serum cystatin C as an indicator of renal function and mortality in liver transplant recipients: Transplantation, 2015; 99; 1431-35
13. Llauger L, Jacob J, Miró Ò, Renal function and acute heart failure outcome: Med Clin (Barc), 2018; 151; 281-90
14. Beddhu S, Samore MH, Roberts MS, Creatinine production, nutrition, and glomerular filtration rate estimation: J Am Soc Nephrol, 2003; 14; 1000-5
15. Wu CK, Lin JW, Caffrey JL, Cystatin C and long-term mortality among subjects with normal creatinine-based estimated glomerular filtration rates: NHANES III (Third National Health and Nutrition Examination Survey): J Am Coll Cardiol, 2010; 56; 1930-36
16. Yang Y, Kim KY, Hwang I, Cystatin C-based equation for predicting the glomerular filtration rate in kidney transplant recipients: Transplant Proc, 2017; 49; 1018-22
17. Hošková L, Franekova J, Málek I, Comparison of cystatin C and NGAL in early diagnosis of acute kidney injury after heart transplantation: Ann Transplant, 2016; 21; 329-45
18. Magnusson M, Hedblad B, Engström G, High levels of cystatin C predict the metabolic syndrome: the prospective Malmö Diet and Cancer Study: J Intern Med, 2013; 274; 192-99
19. Vallianou NG, Georgousopoulou E, Evangelopoulos AA, Inverse relationship between adherence to the Mediterranean diet and serum cystatin C levels: Cent Eur J Public Health, 2017; 25; 240-44
20. Ludwig-Borycz E, Guyer HM, Aljahdali AA, Baylin A, Organic food consumption is associated with inflammatory biomarkers among older adults: Public Health Nutr, 2021; 24; 4603-13
21. Sabbatini M, Ferreri L, Pisani A, Nutritional management in renal transplant recipients: A transplant team opportunity to improve graft survival: Nutr Metab Cardiovasc Dis, 2019; 29; 319-24
22. Sotomayor CG, Gomes-Neto AW, Eisenga MF, Consumption of fruits and vegetables and cardiovascular mortality in renal transplant recipients: A prospective cohort study: Nephrol Dial Transplant, 2020; 35; 357-65
23. Cai Q, Osté MCJ, Gomes-Neto AW, Metabolic syndrome-related dietary pattern and risk of mortality in kidney transplant recipients: Nutr Metab Cardiovasc Dis, 2021; 31; 1129-36
24. Jongbloed F, De Bruin RWF, Steeg HV, Protein and calorie restriction may improve outcomes in living kidney donors and kidney transplant recipients: Aging (Albany NY), 2020; 12; 12441-67
25. Zhao H, Andreyeva T, Diet quality and health in older Americans: Nutrients, 2022; 1; 1198
26. Bishop N, Zhu J, A Prospective cohort study of racial/ethnic variation in the association between change in cystatin C and dietary quality in older Americans: Br J Nutr, 2022; 11; 1-30
27. Echouffo-Tcheugui JB, Ahima RS, Does diet quality or nutrient quantity contribute more to health?: J Clin Invest, 2019; 129; 3969-70
28. He Y, Fang YH, Xia J, Revision of the Chinese dietary balance index: DBI-16: Journal of Nutrition, 2018; 40; 526-30
29. Rimm EB, Giovannucci EL, Stampfer MJ, Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals: Am J Epidemiol, 1992; 135; 1114-26
30. Zang J, Yu H, Zhu Z, Does the dietary pattern of shanghai residents change across seasons and area of residence: Assessing dietary quality using the Chinese Diet Balance Index (DBI): Nutrients, 2017; 9; 251
31. He D, Qiao Y, Xiong S, Association between dietary quality and prediabetes based on the Diet Balance Index: Sci Rep, 2020; 10; 3190
32. Shang H, Wang YS, Shen ZY: National protocol for the conduct of clinical testing, 2014, People’s Medical Publishing House
33. Antonio JP, Sarmento RA, De Almeida JC, Diet quality and glycemic control in patients with type 2 diabetes: J Acad Nutr Diet, 2019; 119; 652-58
34. Kshirsagar AV, Kibbe MR, Gerber DA, Transplant first, dialysis last: JAMA Surg, 2019; 154; 991-92
35. Wai SN, Kelly JT, Johnson DW, Campbell KL, Dietary patterns and clinical outcomes in chronic kidney disease: The CKD.QLD Nutrition Study: J Ren Nutr, 2017; 27; 175-82
36. Silva MTD, Diniz MFHS, Coelho CG, Intake of selected foods and beverages and serum uric acid levels in adults: ELSA-Brasil (2008–2010): Public Health Nutr, 2020; 23; 506-14
37. Herber-Gast GM, Biesbroek S, Verschuren WM, Association of dietary protein and dairy intakes and change in renal function: Results from the population-based longitudinal Doetinchem cohort study: Am J Clin Nutr, 2016; 104; 1712-19
38. Neto AWG, Boslooper-Meulenbelt K, Geelink M, Protein intake, fatigue and quality of life in stable outpatient kidney transplant recipients: Nutrients, 2020; 12; 2451
39. Pedrollo EF, Nicoletto BB, Carpes LS, Effect of an intensive nutrition intervention of a high protein and low glycemic-index diet on weight of kidney transplant recipients: Study protocol for a randomized clinical trial: Trials, 2017; 18; 413
40. Gaipov A, Jackson CD, Talwar M, Association between serum prealbumin level and outcomes in prevalent kidney transplant recipients: J Ren Nutr, 2019; 29; 188-95
41. Toffaletti JG, Hammett-Stabler C, Handel EA, Effect of beef ingestion by humans on plasma concentrations of creatinine, urea, and cystatin C: Clin Biochem, 2018; 58; 26-31
42. Schmitt R, Bachmann S, Impact of thyroid dysfunction on serum cystatin C: Kidney Int, 2003; 64(3); 1139-40
43. Nolte Fong JV, Moore LW, Nutrition trends in kidney transplant recipients: The importance of dietary monitoring and need for evidence-based recommendations: Front Med (Lausanne), 2018; 5; 302
44. Lin IH, Duong TV, Nien SW, Dietary diversity score: Implications for obesity prevention and nutrient adequacy in renal transplant recipients: Int J Environ Res Public Health, 2020; 17; 5083
45. Zhong VW, Ning H, Van Horn L, Diet quality and long-term absolute risks for incident cardiovascular disease and mortality: Am J Med, 2021; 134; 490-98
46. Gillis KA, Patel RK, Jardine AG, Cardiovascular complications after transplantation: Treatment options in solid organ recipients: Transplant Rev (Orlando), 2014; 28; 47-55
Tables
 Table 1. Characteristics of the study participants as stratified by the serum cystatin C index.
Table 1. Characteristics of the study participants as stratified by the serum cystatin C index. Table 2. Scores for the DBI-16 components and the percentages of participants with each score in the 2 groups.
Table 2. Scores for the DBI-16 components and the percentages of participants with each score in the 2 groups. Table 3. Distribution of DBI-16 indicators among the participants.
Table 3. Distribution of DBI-16 indicators among the participants. Table 4. A comparison of component DBI-16 scores between the groups.
Table 4. A comparison of component DBI-16 scores between the groups. Table 5. Subject Work Characteristics (ROC) curve results.
Table 5. Subject Work Characteristics (ROC) curve results. Table 6. A logistic regression model of dietary quality and cystatin C in renal transplant recipients.
Table 6. A logistic regression model of dietary quality and cystatin C in renal transplant recipients. Table 7. Correlation analysis between components of DBI-16 and eGFR based on serum cystatin C(r).
Table 7. Correlation analysis between components of DBI-16 and eGFR based on serum cystatin C(r). In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








