26 September 2023: Original Paper
Dynamic Changes of Regulatory T Cells/CD4 T Cells in Peripheral Blood of Adult Kidney Transplant Recipients: A Comparison of Pediatric and Adult Kidney Donors
Qi Xiao1ABCDEFG, Zide Chen1ABCDEF, Shitao Zhao1BCD, Kaifeng Luo1BCD, Fuping Cao1BF, Zexu Zhang1BF, Jia Liu1BF, Jiansheng Xiao1ACDEFG*DOI: 10.12659/AOT.940604
Ann Transplant 2023; 28:e940604
Abstract
BACKGROUND: Inducing transplantation tolerance and monitoring the recipient’s immune status to improve allograft survival remains the main goal for kidney transplantation (KTx).
MATERIAL AND METHODS: A total of 53 renal transplantation patients and 20 healthy individuals were assigned to the post-transplantation and healthy groups, respectively; 10 recipients with stable renal function for 2 years after kidney transplantation were assigned to Group C. Eleven kidney transplantation recipients were hospitalized due to lung infection. Flow cytometry was used to measure levels of Tregs/CD4⁺ T cells.
RESULTS: The Tregs/CD4⁺ T cells ratio reached homeostasis 6 months after KTx, with no significant difference between Group D (healthy control group) and pre-surgery or Group C (2 years after KTx group). The pediatric donor group and the adult donor group reached immune homeostasis 3 months after the operation. Immune homeostasis is maintaining a balance between immune tolerance and immunogenicity. There was no significant difference in graft function between the pediatric and adult donor groups before surgery, 1 day after surgery, 1 week after surgery, 2 weeks after surgery, and 1 month after surgery; however, graft function was significantly better in the pediatric donor group compared with the adult donor group at 3 mouths (eGFR: 51.7 (40.4-66.2) vs 73.0 (55.7-90.2), P=0.008<0.05) and 6 months (eGFR: 52.2 (37.5-62.8) vs 80.5 (64.1-90.4), P<0.001) after surgery. Pediatric donor kidneys reached immune homeostasis 3 months after surgery, with better graft function at this time compared with adult donor kidneys. The proportion of Tregs/CD4⁺ T cells in recipients with a pulmonary infection after KTx was lower than in those with infection recovery.
CONCLUSIONS: Expanding the use of pediatric kidneys should be further explored by the transplantation community. The proportion of Tregs/CD4⁺ T cells in recipients with a pulmonary infection after KTx was lower than in those with infection recovery.
Keywords: Kidney Transplantation, T-Lymphocytes, Regulatory, Respiratory Tract Infections, Donor Selection, Humans, Adult, Child, CD4-Positive T-Lymphocytes, transplant recipients, Tissue Donors
Background
Kidney transplantation is the best treatment option for patients with end-stage renal disease, and leads to significant improvements in morbidity and mortality [1]. Graft survival and the future function of a kidney transplant correlate with the kidney recipient’s immune status and sensitization, organ quality, and immunosuppressive treatment. Moreover, successful kidney allograft survival may depend on the need for immunosuppressants that are associated with numerous toxicities, including nephrotoxicity [2,3]. Even long-surviving grafts are at risk of chronic allograft nephropathy [4], which may be a form of chronic rejection [5].
Inducing transplantation tolerance and monitoring the recipient’s immune status to improve allograft survival remains the main goal for kidney transplantation. Transplant tolerance is expected to be an effective tool for reducing rejection and minimizing immunosuppressive adverse effects to improve graft outcome [6]. Tolerance induction in allogenic donor grafts is achieved by altering T cell-dependent immune responses via regulatory T cells generation and alloreactive T cell reduction [7].
In recent years, clinical research has focused on regulatory T cells (Tregs) that help prevent initial priming of memory alloreactive T cells and are involved in the induction of allograft tolerance [8]. CD4+CD25+ Tregs, a distinct lineage of CD4+ T cells, are crucial for negatively regulating immune responses and are generally considered to be important regulators of immune tolerance [7,9]. Currently, the major marker of Tregs is the transcription factor (FoxP3); however, FoxP3 cannot be used to separate human Tregs for functional studies or in vivo expansion for cell therapy as an intracellular protein. To isolate Tregs, IL-7R (CD127), the α-chain of the IL-17 receptor, is a replacement for FoxP3, as the relative expression of CD127 is inversely correlated with FoxP3, with the highest FoxP3-expressing CD4+ T cells showing the lowest levels of CD127. In addition, CD127 is a better marker than CD25 because all CD4+ T cells are CD127low/− independent of CD25 expression [10]. Phenotypically, Tregs may be defined CD4+CD25+ CD127low/− [11,12].
Tregs exert their suppressive function directly by surface-expressed molecules (eg, CTLA4, GITR, and OX40) and indirectly by the secretion of anti-inflammatory cytokines, mainly including transforming growth factor (TGF-β1) and interleukin (IL)-10 [13]. Studies have found that specific immunosuppression can be maintained by adding endogenous Tregs or using exogenous Tregs in a dose-dependent manner, to achieve immune tolerance or reduce complications caused by long-term immunosuppression after kidney transplantation [14,15]. Krajewska et al [8] found that the expression levels of peripheral blood regulatory T cell-related genes are associated with long-term survival in renal transplantation patients, and Trovillion et al [16] found that post-transplantation regulatory T cells are associated with chronic graft-versus-host disease. Therefore, it has been speculated that Tregs in the lymphatic tissue effectively inhibit the immune system from attacking the graft. The number of Tregs in recipients with chronic rejection was found to be significantly lower than in recipients with long-term stable graft function [17,18]. Opportunistic infections in renal transplantation recipients, total lymphocytes, CD3+ T lymphocytes, and CD4+ T lymphocytes were significantly decreased in the 6th month after the operation [19]. The number of CD4+ T lymphocytes in the first month after surgery was the most predictive parameter of the occurrence of opportunistic infection [20]. Studies found that pneumocystis pneumonia after kidney transplantation is closely related to the downregulation of Tregs [21,22].
The incidence of acute rejection is high in the early stage of adult kidney transplantation from pediatric donors [23–25], which can be reduced through optimization of immunosuppression, including induction therapy [26,27] and steroid-based immunosuppression [23,27]. In pediatric donor kidney transplantation, acute rejection is not only related to the selected immunosuppression regimen, but also may be related to the specificity of the pediatric donor kidney [28,29]. Tulane University [30] reported that when children provide transplant kidneys for adults, the incidence of acute rejection is as high as 25%; the possible reasons may be postoperative transplantation renal insufficiency and high risk during the filling, and increased damage causing inflammation, as well as HLA antigen expression for the kidney.
However, to date, there have been no published studies on adult kidney transplantation comparing pediatric and adult donors. Immune homeostasis is maintaining a balance between immune tolerance and immunogenicity. In this study, we compared immune homeostasis of recipients of kidneys from pediatric vs adult donors. Secondly, we try to clarify the relationship between Tregs/CD4+ T cells ratio and immune homeostasis. Thirdly, we sought to validate previous findings of the relationship between pulmonary infection and peripheral blood Tregs/CD4+ T cells ratio.
Material and Methods
STUDY POPULATION:
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. The study was conducted in accordance with the Istanbul Declaration. Uremia patients with the need for transplantation who provided signed informed consent were enrolled. All kidney allografts conformed with standard criteria for deceased donors. Peripheral blood samples from kidney transplant recipients were collected in the Organ Transplantation Department of the First Affiliated Hospital of Nanchang University from May 2020 to July 2021. There were 53 patients, including 32 males and 21 females. There were 35 adult and 18 pediatric donors, with an average age of 39.4±11.4 years. Twenty healthy controls undergoing medical examination (9 males and 11 females, aged 47.4±8.8 years) were selected from the First Affiliated Hospital of Nanchang University. All participants were informed and consented to participate in the study. The endpoints included graft loss and recipient death, for up to 6 months. The analysis also included 10 recipients (7 males and 3 females, aged 38.9±8.5 years) treated for 2 years with stable renal function since kidney transplantation. Treg levels at various time points after surgery were compared with the group 2 years after KTx to determine when patients reached immune homeostasis after surgery. Basiliximab was used for induction, and all patients received conventional immunosuppressive drugs, including tacrolimus, mycophenolate mofetil, and steroids.
ELIGIBILITY CRITERIA:
Inclusion criteria were: end-stage renal disease, first-time recipient of kidney transplantation, negative preoperative lymph node toxicity, ≥18 years old and ≤70 years old, and signed informed consent. Exclusion criteria were: irregular postoperative follow-up and no complete follow-up data, receiving double kidney transplantation, receiving second kidney transplantation, and ABO blood group incompatibility or positive crossmatch.
SPECIMEN COLLECTION:
Under sterile conditions, about 10 ml of peripheral blood (PB) was collected per patient at 7 time points: the day before KTx (D0), the first day after KTx (D1), the first-week after KTx (W1), the second-week after KTx (W2), and at month 1 (M1), month 3 (M3), and month 6 (M6). Ten-mL heparin anticoagulant tubes were used for flow cytometry detection. Kidney function was assessed as estimated glomerular filtration rate (eGFR) and calculated with the Modification of Diet in Renal Disease (MDRD) study formula.
FLUORESCENCE-ACTIVATED CELL SORTING:
Ten mL of venous blood from each subject was divided into 2 ethylenediaminetetraacetic acid disodium salt (EDTA) anticoagulant tubes. Peripheral blood mononuclear cells were separated by lymphocyte stratification solution density gradient centrifugation, and the cell density was adjusted to 1×109/L for flow cytometry analysis. All samples were collected within 8 h and tested as follows. In total, 50 μl of peripheral blood was collected and supplemented with CD4-Fluorescein isothiocyanate (FITC), CD25-PC5, and CD127-Phycoerythrin (PE) (BD Pharmingen, San Diego, USA). After incubation at 4°C for 30 min away from light, hemolysin was added for 15 min at 4°C away from light. The cells were washed with 2 ml PBS by centrifugation at 1500 rpm for 10 min, and resuspended in 500 μl PBS. CD4+CD25+CD127− T cells were analyzed by flow cytometry on a CTYOMICS FC 500. Electronic gates were set on the CD4+ T cells, then we set the electronic gates at CD25+CD127−T cells based on the CD4+ T cells. CD4+CD25+CD127− cells were considered as Treg cells. The gating strategy is shown in Figure 1.
STATISTICAL METHODS:
The SPSS 26.0 statistical analysis software was used to analyze the clinical parameters and laboratory indicators. M (Q25, Q75) and mean±SD (
Results
CLINICAL DATA:
A total of 53 subjects were followed up, including 32 males and 21 females. There were 35 adult and 18 pediatric donors. Blood samples were obtained from prospective KTx recipients preoperatively (eGFR in ml/min/1.73 m2: median, 4.8; IQR, 3.9–6.6), 1 day after KTx (eGFR: median, 5.2; IQR, 4.3–7.2), 1 week after KTx (eGFR: median, 24.2; IQR, 10.4–49.2), 2 weeks after KTx (eGFR: median, 36.2; IQR, 15.7–67.7), 1 month after KTx (eGFR: median, 47.5; IQR, 36.2–68.5), 3 months after KTx (eGFR: median, 57.2; IQR, 44.0–73.1), and 6 months after KTx (eGFR: median, 58.0; IQR, 45.1–76.5). No significant correlation was observed between dialysis time and transplanted renal function (eGFR). Donor age was negatively correlated with allograft function at M1 (rs=−0.273, P=0.048), M3 (rs=−0.328, P=0.017) and M6 (rs=−0.544, P=0.000). Also, recipient age was negatively correlated with allograft function at M1 (rs=−0.320, P=0.020), M3 (rs=−0.366, P=0.007), and M6 (rs=−0.305, P=0.026). The Tregs/CD4+ T cell ratio was positively correlated with graft function at M3 (rs=0.408, P=0.002). Lymphocyte count was positively correlated with allograft function at W2 (rs=0.422, P=0.002), M1 (rs=0.272, P=0.049) and M3 (rs=0.288, P=0.037). Detailed factors associated with estimated glomerular filtration rate (with correlation coefficients and P values) are provided in Tables 1 and 2.
:
After kidney transplantation, Tregs/CD4+ T cells ratios were significantly reduced, even reaching 0%. One month after KTx, the Tregs/CD4+ T cells ratio decreased to the lowest value and then gradually increased; it was 96.23% lower than the preoperative value. Except for 6 months after KTx, Tregs/CD4+ T cells ratios were significantly lower than those obtained before KTx. The dynamic changes in peripheral blood Tregs-to-CD4+ T cells ratio are provided in Figure 2. The Tregs-to-CD4+ T cells ratio of the group 2 years after KTx (group C) and the healthy control group (group D) are provided in Figure 3. There was no significant difference between Tregs/CD4+ T cells ratios before KTx and at 6 months after KTx (P>0.05). Although Tregs/CD4+ T cells ratios at 6 months after KTx were still lower than those obtained at 2 years after KTx (group C) and in the healthy control group (group D) (median, 0.0430 [IQR=0.0285–0.0555], 0.0600 [IQR=0.0575–0.0700] and 0.0700 [IQR=0.0600–0.0800], respectively; P=1.00, P=0.193), no significant difference was found among them. Moreover, Tregs/CD4+ T cells ratios 2 years after KTx were not significantly different from those before KTx and group D values (P>0.05). There was no significant difference between pre-KTx and group D values (P>0.05). At the same time, lymphocyte count was immediately decreased after KTx and reached the lowest level at 1 day after KTx. Lymphocyte count at 1 day after KTx was decreased by 64.5% compared with the pre-KTx value (median, 1.10 [IQR=0.93–1.44] vs 0.39 [IQR=0.31–0.51]; P<0.001), and lower than in Groups C (median, 1.92 [IQR=1.37–2.29]) and D (median, 1.51 [IQR=1.31–1.65]), also with statistically significant differences (both P<0.01). Lymphocyte count was increased gradually and reached the highest value 3 months after KTx. The dynamic changes of lymphocyte count are shown in Figure 4. Six months after KTx, the lymphocyte count was still higher than the pre-KTx value, and there was a statistically significant difference between the pre-KTx lymphocyte count and at 6 months after KTx (median, 1.10 [IQR=0.93–1.44] vs 1.89 [IQR=1.36–2.51]; P<0.01). Meanwhile, there was no significant difference in lymphocyte count between Group C and Group D and at 6 months after KTx (median, 1.92 [IQR=1.37–2.290, 1.51 [IQR=1.31–1.65] and 1.89 [IQR=1.36–2.51], respectively; P>0.05, P>0.05). Tregs/CD4+ T cells ratios and lymphocyte counts (median [Q25, Q75]) are provided in Table 3.
DIFFERENCES IN CLINICAL INDEXES AFTER RENAL TRANSPLANTATION BETWEEN THE PEDIATRIC AND ADULT DONOR GROUPS:
The detailed characteristics of transplant recipients and donors are provided in Table 4. There were no significant differences in eGFR at 0 day (D0), 1 day (D1), 1 week (W1), 2 weeks (W2), and 1 month (M1). At 3 and 6 months after KTx, eGFR in the pediatric donor group was significantly higher than that of the adult donor group. The detailed eGFRs at various time points are provided in Table 5. At 3 months after KTx, median (IQR) eGFR in the pediatric donor group was 73.0 (55.7–90.2), vs 51.7 (40.4–66.2) in the adult donor group, indicating a significant difference between the 2 groups (P=0.008). At 6 months after kidney transplantation, there was also a significant difference in renal function between the 2 groups (P<0.001). The median (IQR) eGFR in the pediatric kidney donor group was 80.5 (64.1–90.4) vs 52.2 (37.5–62.8) in the adult kidney donor group.
There were no significant differences in creatinine levels at D0, D1, W1, and W2. There were significant differences between the 2 groups at 1, 3, and 6 months after KTx (P<0.05). Creatinine levels were lower in the pediatric donor group compared with the adult donor group at 1, 3, and 6 months after KTx. The detailed creatinine levels at various time points are provided in Table 5. Median (IQR) creatinine levels in the pediatric and adult donor groups were 113.0 (94.3–154.5) and 138.9 (114.2–185.1) at 1 month after KTx, respectively (P=0.046). Median (IQR) creatinine levels in the pediatric and adult donor groups were 91.4 (76.1–115.6) and 129.0 (105.3–158.1) at 3 months after KTx, respectively (P=0.001). At 6 months after KTx, median (IQR) creatinine levels were 88.6 (76.4–105.6) and 133.9 (109.8–155.4), respectively (P=0.000).
There were no significant differences in urea nitrogen (BUN) levels at D0, D1, W1, W2, and M1. There were significant differences between the 2 groups at 3 and 6 months after KTx (P<0.05), with lower urea nitrogen levels in the pediatric donor group compared with the adult donor group. The detailed BUN levels at various time points are provided in Table 5. Median (IQR) urea nitrogen levels in the pediatric and adult donor groups were 6.0 (5.0–7.7) and 7.8 (5.8–9.8) at 3 months after surgery, respectively; at 6 months postoperatively, they were 5.9 (5.0–6.8) and 7.8 (5.6–10.7), respectively.
There were no significant changes in hemoglobin levels at any time point during renal transplantation. There were no significant differences in anastomotic artery resistance index between the pediatric and adult donor groups after renal transplantation.
:
No significant differences were found in Tregs/CD4+ T cells ratios at all time points (before and after transplantation) between the pediatric and adult donor groups (P>0.05), and no significant difference in the lymphocyte count between the 2 groups (P>0.05). The Tregs/CD4+ T cells ratio at various time point between kidney recipients from pediatric and adult donors are provided in Figure 5. There were no significant differences in Tregs/CD4+ T cells ratios at 3 months, 6 months, and 2 years after KTx in the pediatric donor group (P>0.05), as well as at 3 months after KTx compared to pretreatment and control (healthy individuals) values (P>0.05). There were no significant differences in Tregs/CD4+ T cells ratios at 3 months, 6 months, and 2 years after KTx in the adult donor group (P>0.05). The detailed Tregs/CD4+ T cells ratios in the pediatric and adult donor groups are provided in Table 6.
:
We included 11 kidney transplantation recipients hospitalized due to lung infection. Most patients (81.8%) developed pulmonary infection within the first year after transplantations, including 3 cases (27.3%) within the first 3 months. The median time was 6 months. The proportion of Tregs/CD4+ T cells in recipients with a pulmonary infection after KTx was lower than in those with infection recovery. The ratio of Tregs/CD4+ T cells at infection and at infection recovery was 0.025 (0.012–0.035) and 0.046 (0.022–0.056), respectively, which was increased by 84%. There was a significant difference between infection and infection recovery (P=0.007 <0.05). A detailed comparison of Treg/CD4+ T cell in infection and infection recovery is provided in Figure 6.
Discussion
We analyzed immune cells before and during the 6 months following kidney transplantation in stable allograft recipients. Studies have shown that after transplantation, Tregs not only exist in the lymphoid tissue of the recipient, but also appear at the transplantation site [31,32]. This distribution forms 2 channels of protection against immune damage in kidney transplant recipients. Tregs among lymphocytes first effectively prevent effector T cells from attacking the graft, while those that escape from lymphocytes and the graft provide a second channel of protection for the graft. The gene expressions of peripheral blood Tregs and lymphocytes are consistent after transplantation. This study showed that after transplantation, the Tregs/CD4+ T cells ratio in peripheral blood was sharply decreased, even to 0%, and reached the lowest value 1 month after surgery, which may be caused by immunosuppressants, partly because Tregs in peripheral blood migrated to the transplanted kidney and distributed to the transplanted kidney, reaching the steady state at 6 months after surgery. It was reported that calcineurin inhibitors (CNIs) inhibit proliferation of Tregs [33]. There was no significant difference in Tregs/CD4+ T cells ratio 6 months after renal transplantation compared with the presurgical value. There were also no significant differences in Tregs/CD4+ T cells ratios at 6 months and 2 years after surgery versus the healthy control group. Massachusetts General Hospital’s investigators found that the tolerant phenotype is associated with increased proportion of CD4+ CD25+ CD127− FOXP3 Tregs during the early post-transplant period [34]. At 6 months after KTx, the Tregs/CD4+ T cells ratio increased significantly. This may indicate that the recipient has reached immune homeostasis 6 months after kidney transplantation.
We observed no significant differences in Tregs/CD4+ T cells ratios between the pediatric and adult donor groups at various time points before and after transplantation (
As shown above, lymphocyte count was positively correlated with graft function at 2 weeks, 1 month, and 3 months postoperatively. Dujardin et al [35] found that occurrence of deep lymphopenia in recipients beyond 1 year after transplantation increases the risk of long-term graft failure, death, and viral infection compared with counterparts with normal lymphocyte count. In lymphopenia, extensive T cell proliferation can occur, especially of activated memory cells, which constitute a barrier to tolerance and are the most effective and aggressive immune cells against the graft [36]. This may explain why lymphocyte count is positively correlated with renal graft function. We also observed that lymphocyte count was decreased immediately after KTx, reaching the lowest level 1 day after KTx. Lymphocyte count was decreased by 64.5% 1 day after KTx compared with the pre-KTx value, and the difference was statistically significant. In addition to high dose of immunosuppressants and basiliximab, the immediate decrease of lymphocytes after KTx may also be related to intraoperative bleeding. Lymphocyte count increased gradually and reached the highest value 3 months after KTx. The incidence of acute rejection varies between 10% and 50% during the first 6 months [37]. In this study, lymphocyte count reached the highest value 3 months after KTx, while immunosuppressive Tregs were still at a low level from 1 month to 3 months after surgery, which may indicate that the recipients are more prone to acute rejection within 3 months after KTx. Six months after KTx, lymphocyte count was still significantly higher than before KTx. Also, pre-KTx lymphocyte count was lower than that of the healthy group. In end-stage renal disease in renal transplant recipients, the most significant T cell dysregulation found in peripheral blood is a decrease in circulating T lymphocytes [38]. Once the kidney transplantation is performed, the immune state of the body changes immediately and uremia is relieved; this might explain why lymphocyte levels are consistently higher than before surgery.
The use of pediatric donor kidneys can significantly expand the donor pool. However, there have been concerns regarding the use of single pediatric donors due to insufficient donor kidney size, vascular embolism, urethral complications, and ultrafiltration injury of the transplanted kidneys [39,40]. It has been reported that compared with adult donor kidneys, pediatric donor kidneys achieve better graft function and longer graft survival after passing the short-term crisis [39–41]. Similar conclusions were also obtained in this study, with no significant differences in recipient creatinine levels and BUN levels between the pediatric and adult donor kidney groups before surgery, 1 day after surgery, 3 days after surgery, 5 days after surgery, 1 week after surgery, and 2 weeks after surgery. Creatinine levels and BUN amounts in the pediatric donor group were significantly lower than those of the adult donor group at 3 and 6 months after KTx. This strongly points to the importance of nonimmunological factors such as “nephron dosing” as a determinant of allograft function. Nghiem et al [42] convincingly showed growth of pediatric en bloc kidney allografts by nearly 2-fold in 3 to 6 months, approaching 3-fold at 6 months and longer. Therefore, the higher eGFRs in pediatric kidney recipients could be explained by such growth as well as the superior functional glomerular reserve. This finding may indicate that children can achieve better graft function than adults 3 months after surgery once the postoperative risk period is passed.
We measured the levels of Tregs/CD4+ T cells prior to immunosuppressive agent adjustment during infection. After an anti-infection treatment, including reducing the use of immunosuppressive drugs and the complete recovery of infection, the immunosuppressive drugs returned to normal use, and we observed the proportion of Tregs/CD4+ T cells in the recipient was significantly higher than at the time of infection, and the difference was statistically significant. Ahmed et al [43] found that the CD4+ T cell count and CD4+/CD8+ ratio of recipients with a postoperative pulmonary infection were significantly lower than those before infection and after the infection was cured, which, if increased significantly, would indicate that the infection had been improved. However, we found that Tregs/CD4+ T cells decreased significantly when infection occurred, which indicated that the decrease in Tregs was much larger than in CD4+ T cells, which may indicate that Tregs combined with CD4+ T cells could better predict infection and infection recovery after kidney transplantation.
Our study has some limitations. This was a single-center study with a small sample size, and many statistical tests were performed without adjustment for multiple hypothesis testing and possible confounders, the study was hypothesis-generating, and the results need to be confirmed in further studies. In addition, it is unknown if the findings can be generalized to populations given a different therapy than basiliximab, and further studies are needed to confirm this hypothesis.
Conclusions
The Tregs/CD4+ T cells ratio in renal transplantation recipients decreases significantly after surgery, and the postoperative immune status changes compared with preoperative and control (healthy subjects) conditions, reaching immune homeostasis at 6 months postoperatively. Once the pediatric donor kidney passes the perioperative period, better graft function is achieved. The time to immune homeostasis of pediatric donor kidneys and adult donor kidneys is the same. Tregs combined with CD4+ T cells could better predict infection and infection recovery after kidney transplantation.
Figures
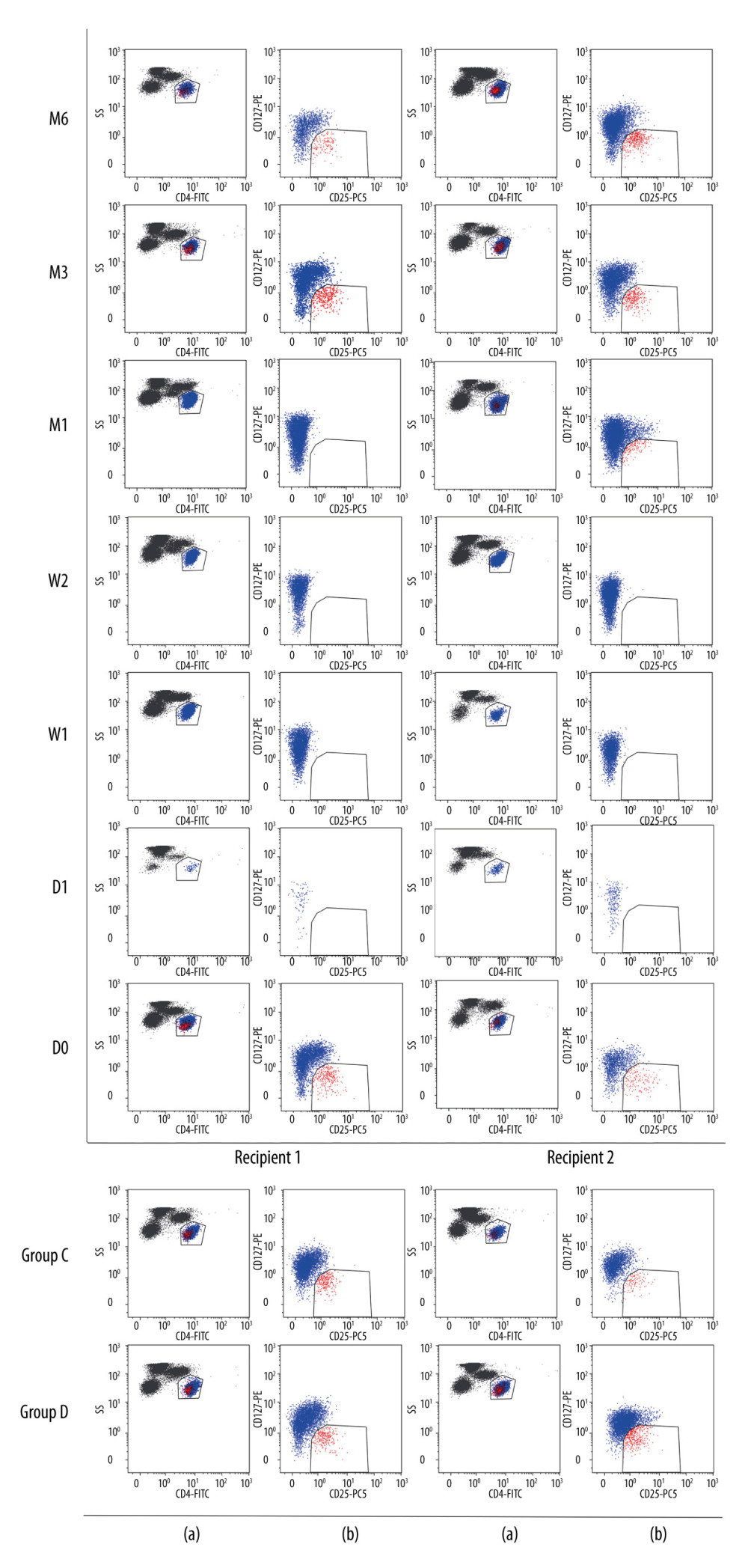 Figure 1. Flow cytometry gating strategy. The lymphocyte population was selected based on FSC/SSC scatters: (a) CD4+ lymphocytes dot plot; (b) CD127 vs CD25 dot plot of CD4+ lymphocytes. D0: the day before KTx, D1: the first day after KTx, W1: the first-week after KTx, W2: the second-week after KTx, M1: the first month after KTx, M3: the third month after KTx, M6: the sixth month after KTx. Group C: 2 individual kidney recipients with stable renal function for 2 years after kidney transplantation. Group D: 2 healthy individuals.
Figure 1. Flow cytometry gating strategy. The lymphocyte population was selected based on FSC/SSC scatters: (a) CD4+ lymphocytes dot plot; (b) CD127 vs CD25 dot plot of CD4+ lymphocytes. D0: the day before KTx, D1: the first day after KTx, W1: the first-week after KTx, W2: the second-week after KTx, M1: the first month after KTx, M3: the third month after KTx, M6: the sixth month after KTx. Group C: 2 individual kidney recipients with stable renal function for 2 years after kidney transplantation. Group D: 2 healthy individuals. 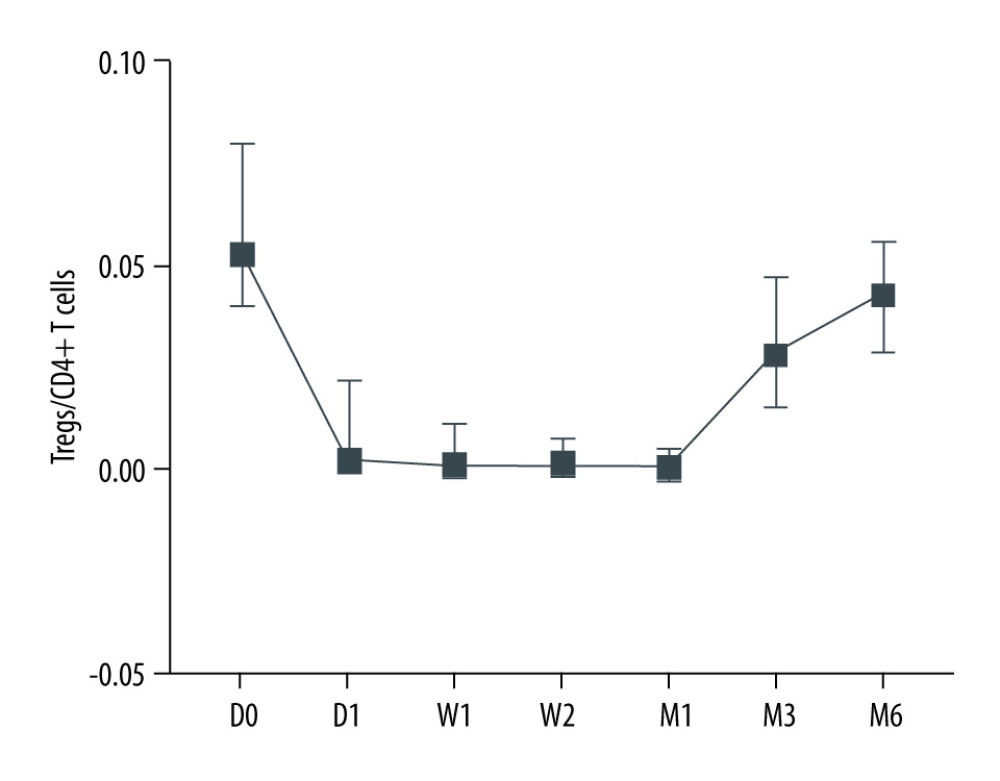 Figure 2. Dynamic changes of Tregs-to-CD4+ T cells ratio.
Figure 2. Dynamic changes of Tregs-to-CD4+ T cells ratio. 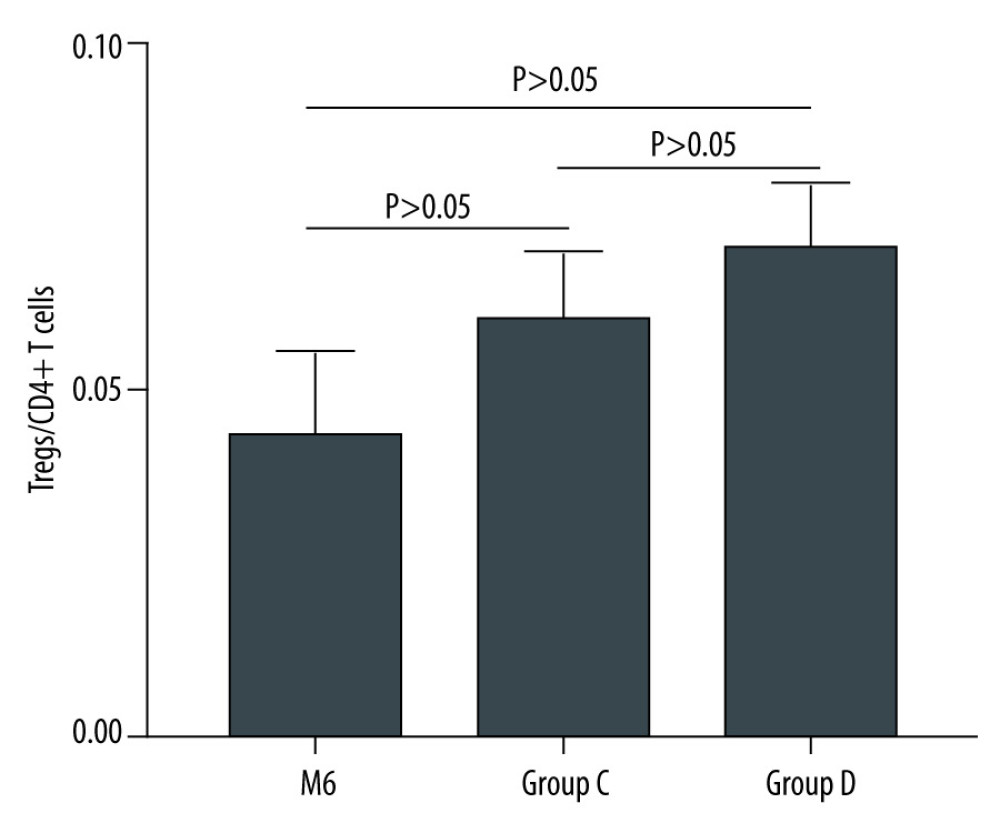 Figure 3. The Tregs-to-CD4+ T cells ratio of groups C and D.
Figure 3. The Tregs-to-CD4+ T cells ratio of groups C and D. 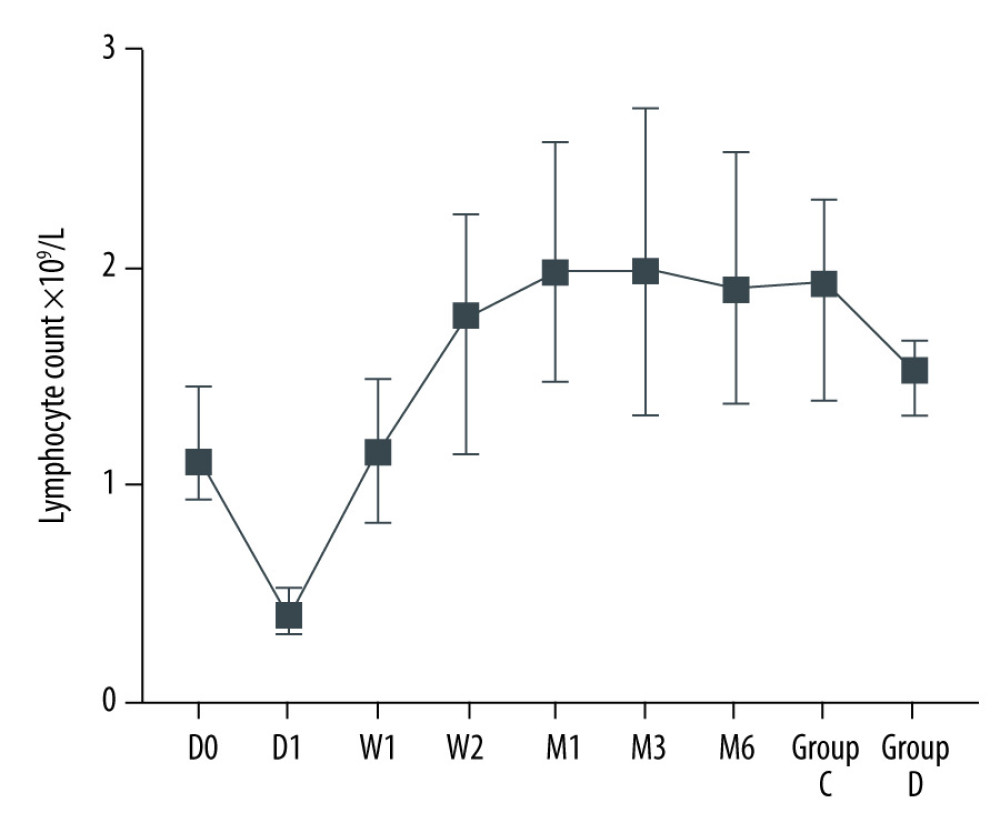 Figure 4. Dynamic changes of lymphocyte count.
Figure 4. Dynamic changes of lymphocyte count. 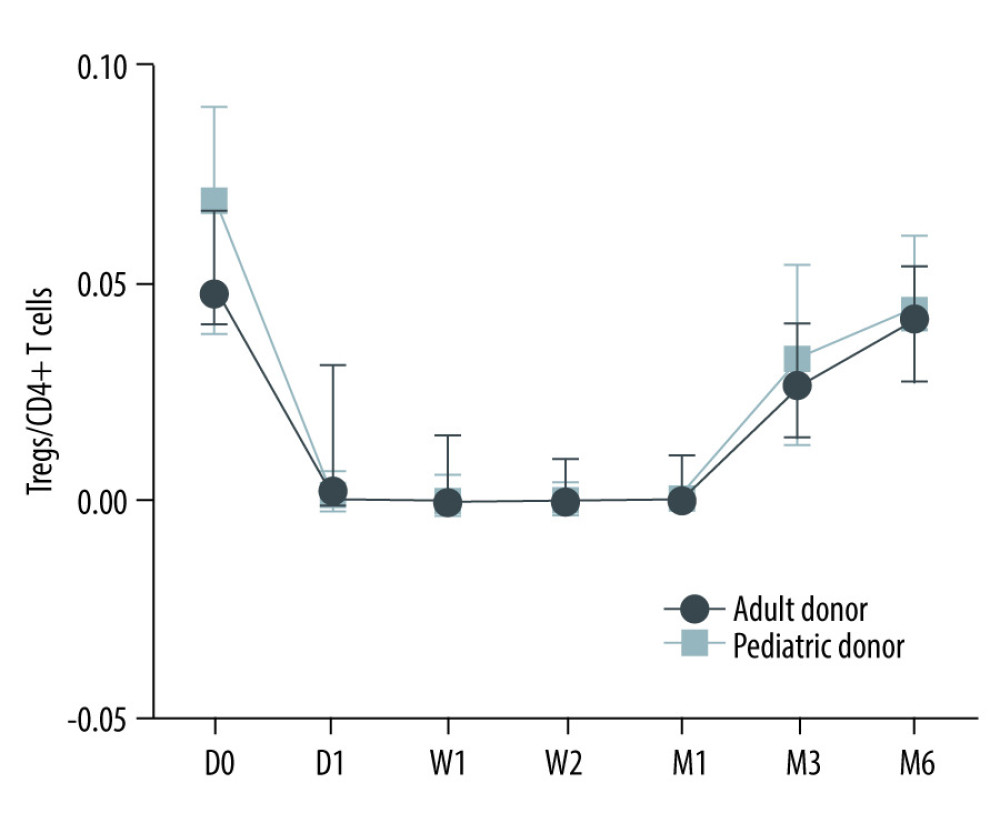 Figure 5. The Tregs/CD4+ T cells ratio at various time point between kidney recipients from pediatric and adult donors.
Figure 5. The Tregs/CD4+ T cells ratio at various time point between kidney recipients from pediatric and adult donors. 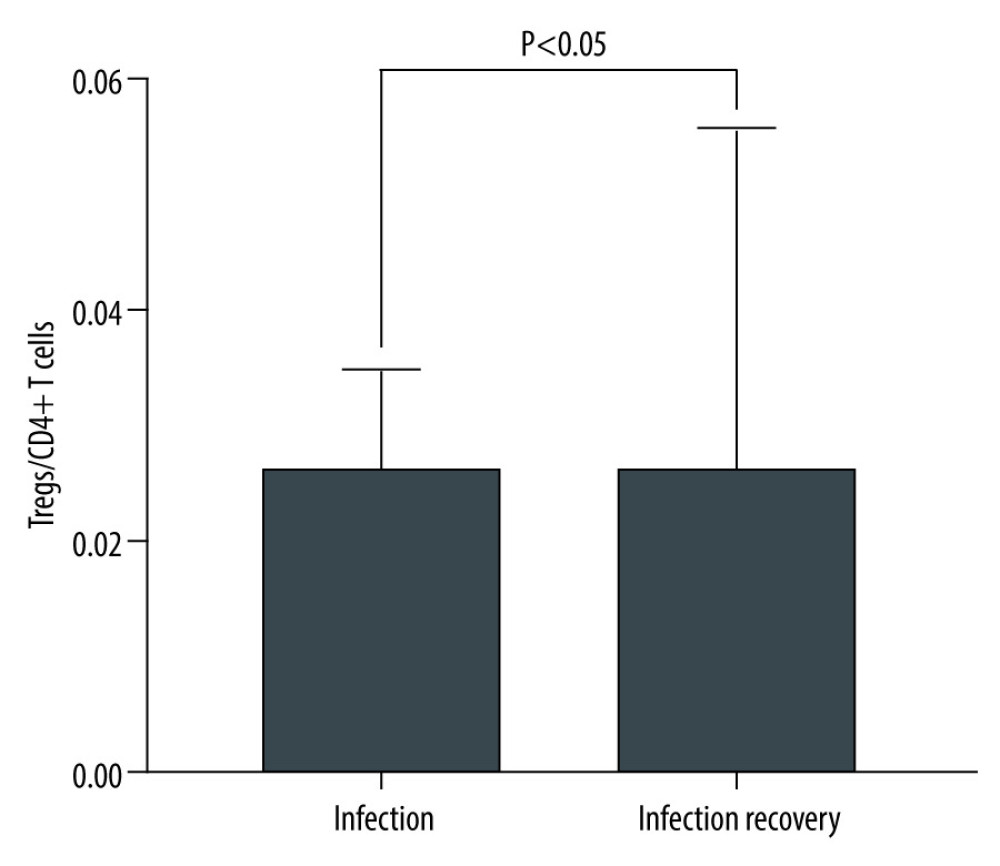 Figure 6. The level of Tregs/CD4+ T cell in infection and infection recovery.
Figure 6. The level of Tregs/CD4+ T cell in infection and infection recovery. Tables
Table 1. Factors positively and negatively correlated with graft function.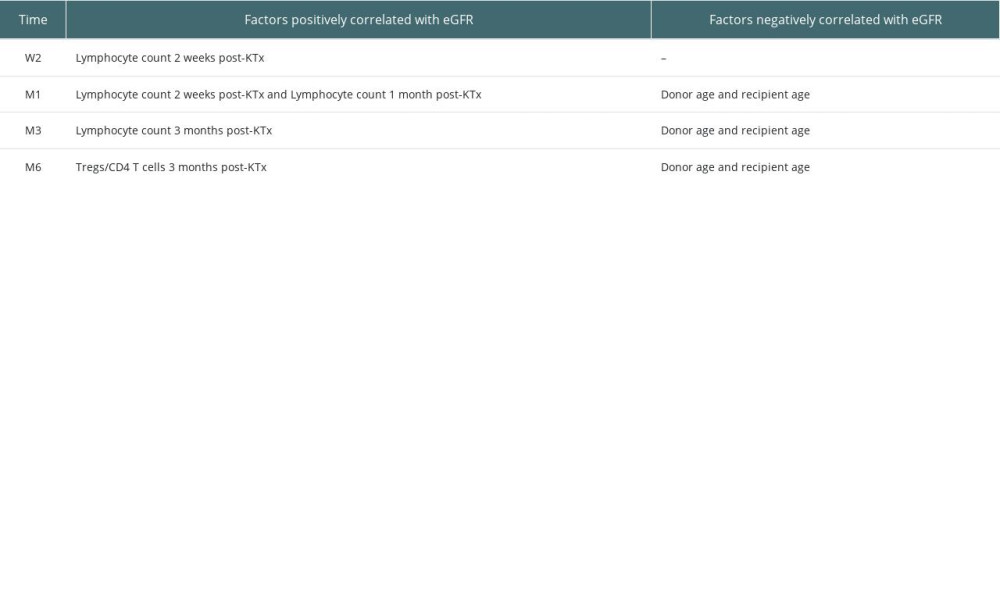 Table 2. Factors associated with estimated glomerular filtration rate (correlation coefficient, p value).
Table 2. Factors associated with estimated glomerular filtration rate (correlation coefficient, p value).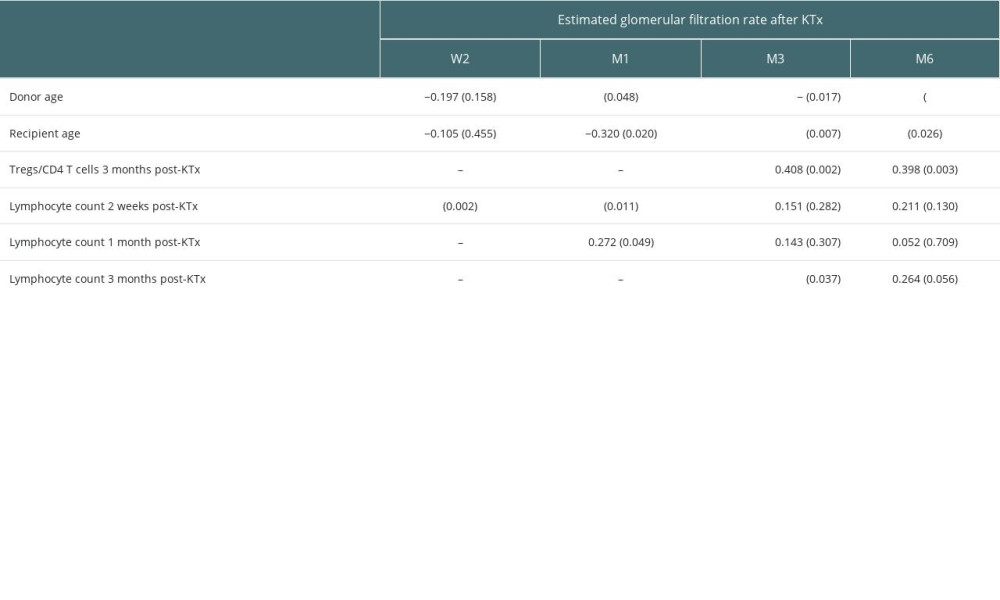 Table 3. Tregs/CD4+ T cells ratios and lymphocyte counts in median (Q25, Q75).
Table 3. Tregs/CD4+ T cells ratios and lymphocyte counts in median (Q25, Q75).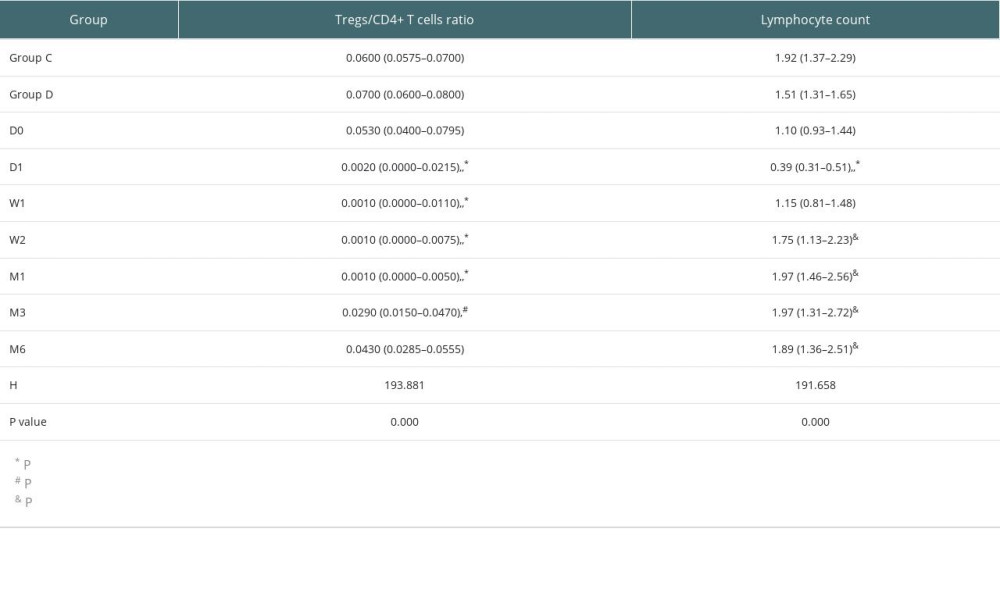 Table 4. Demographic characteristics of transplant recipients and donors.
Table 4. Demographic characteristics of transplant recipients and donors.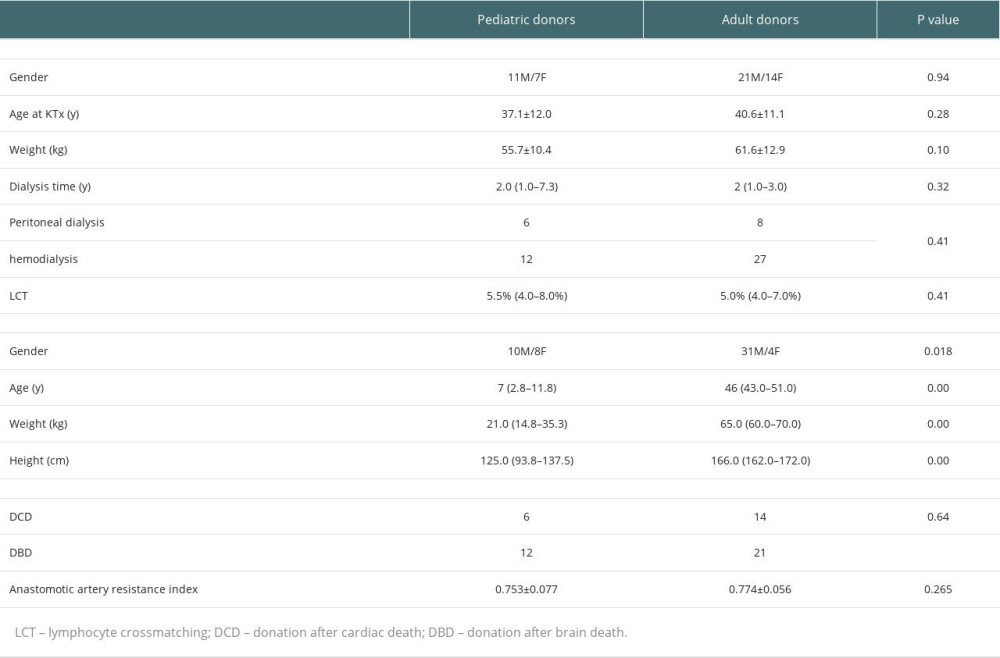 Table 5. Detailed eGFRs, creatinine levels and BUN amounts at various time points.
Table 5. Detailed eGFRs, creatinine levels and BUN amounts at various time points.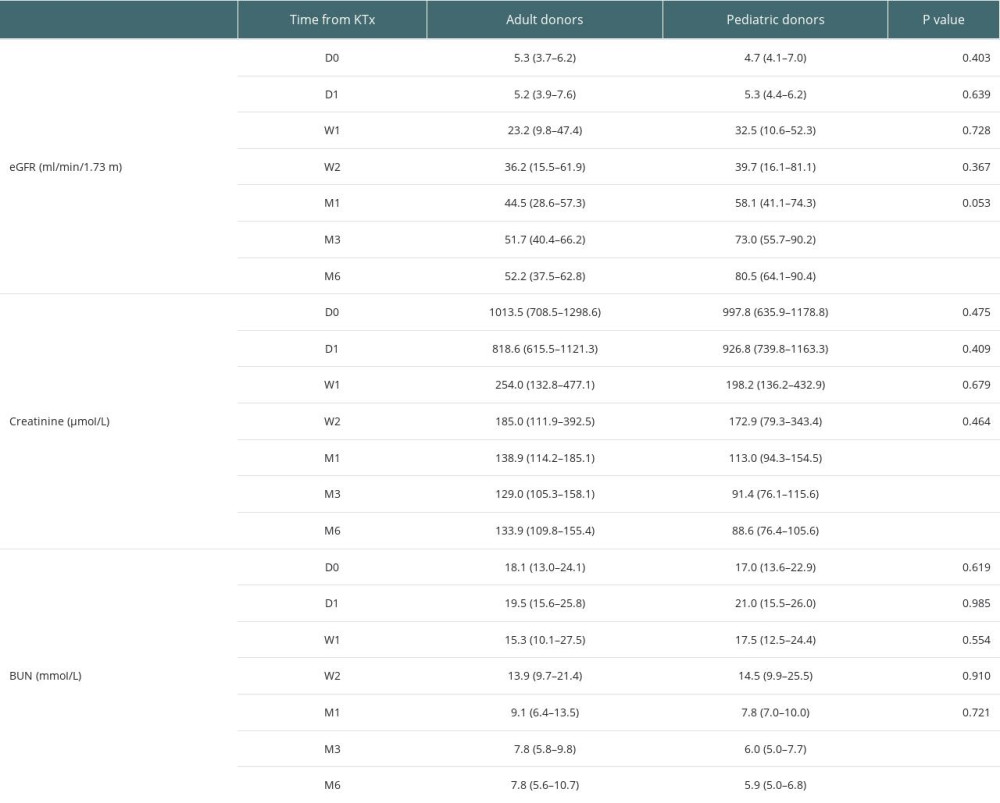 Table 6. Tregs/CD4+ T cells ratios in the pediatric and adult donor groups in median (Q25, Q75).
Table 6. Tregs/CD4+ T cells ratios in the pediatric and adult donor groups in median (Q25, Q75).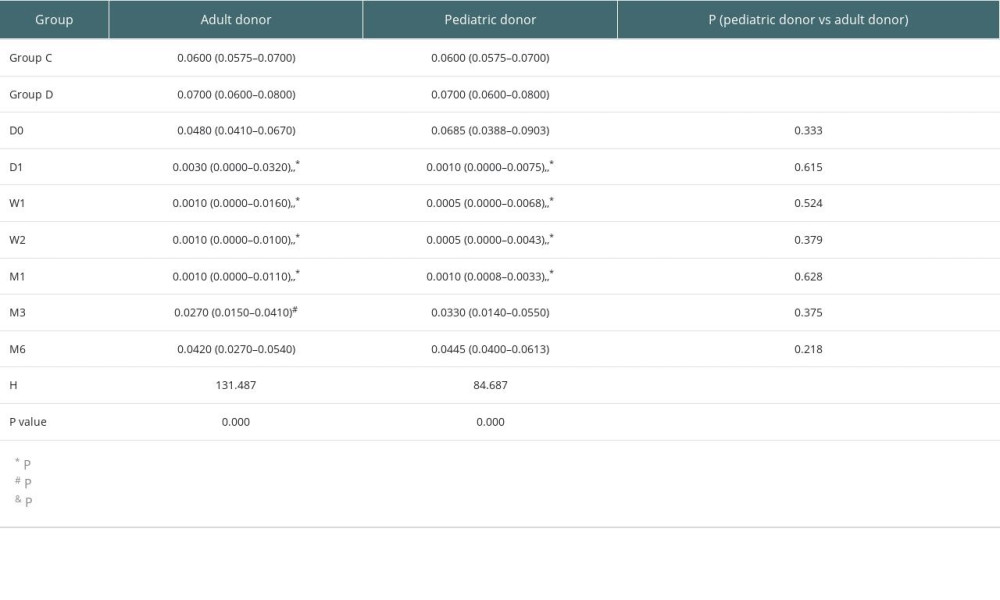
References
1. Flechner SM, Kurian SM, Head SR, Kidney transplant rejection and tissue injury by gene profiling of biopsies and peripheral blood lymphocytes: Am J Transplant, 2004; 4(9); 1475-89
2. Krämer BK, Zülke C, Kammerl MC, Cardiovascular risk factors and estimated risk for CAD in a randomized trial comparing calcineurin inhibitors in renal transplantation: Am J Transplant, 2003; 3(8); 982-87
3. Naesens M, Kuypers DR, Sarwal M, Calcineurin inhibitor nephrotoxicity: Clin J Am Soc Nephrol, 2009; 4(2); 481-508
4. Birnbaum LM, Lipman M, Paraskevas S, Management of chronic allograft nephropathy: A systematic review: Clin J Am Soc Nephrol, 2009; 4(4); 860-65
5. Colvin RB, Hirohashi T, Farris AB, Emerging role of B cells in chronic allograft dysfunction: Kidney Int Suppl, 2010(119); S13-S17
6. Bestard O, Cruzado JM, Mestre M: J Immunol, 2007; 179(7); 4901-9
7. Qu Y, Zhang B, Zhao L: Transpl Immunol, 2007; 17(3); 153-61
8. Krajewska M, Kościelska-Kasprzak K, Kamińska D, Kidney transplant outcome is associated with regulatory T cell population and gene expression early after transplantation: J Immunol Res, 2019; 2019; 7452019
9. Shouval DS, Biswas A, Goettel JA, Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function: Immunity, 2014; 40(5); 706-19
10. Liu W, Putnam AL, Xu-Yu Z: J Exp Med, 2006; 203(7); 1701-11
11. Georgiev P, Charbonnier LM, Chatila TA, Regulatory T cells: The many faces of Foxp3: J Clin Immunol, 2019; 39(7); 623-40
12. Moldenhauer LM, Jin M, Wilson JJ, Regulatory T cell proportion and phenotype are altered in women using oral contraception: Endocrinology, 2022; 163(9); bqac098
13. Eghbal-Fard S, Yousefi M, Heydarlou H, The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia: J Cell Physiol, 2019; 234(4); 5106-16
14. Campbell DJ, Control of regulatory T cell migration, function, and homeostasis: J Immunol, 2015; 195(6); 2507-13
15. Li X, Zheng Y, Regulatory T cell identity: formation and maintenance: Trends Immunol, 2015; 36(6); 344-53
16. Trovillion EM, Gloude NJ, Anderson EJ, Morris GP, Relationship of post-transplant thymopoiesis with CD4FoxP3 regulatory T cell recovery associated with freedom from chronic graft versus host disease: Bone Marrow Transplant, 2019; 54(6); 917-20
17. Iglesias-Escudero M, Sansegundo-Arribas D, Riquelme P, Myeloid-derived suppressor cells in kidney transplant recipients and the effect of maintenance immunotherapy: Front Immunol, 2020; 11; 643
18. Mirzakhani M, Shahbazi M, Akbari R, Reduced CD4 CD25 CD45RA Foxp3 activated regulatory T cells and its association with acute rejection in patients with kidney transplantation: Transpl Immunol, 2020; 60; 101290
19. Fernández-Ruiz M, López-Medrano F, Allende LM, Kinetics of peripheral blood lymphocyte subpopulations predicts the occurrence of opportunistic infection after kidney transplantation: Transplant Int, 2014; 27(7); 674-85
20. Schürmann M, Schürmann D, Schindler R: Transpl Immunol, 2013; 28(4); 159-63
21. McKinley L, Logar AJ, McAllister F, Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia: J Immunol, 2006; 177(9); 6215-26
22. Hori S, Carvalho TL, Demengeot J: Eur J Immunol, 2002; 32(5); 1282-91
23. Balachandran VP, Aull MJ, Goris M, Successful transplantation of single kidneys from pediatric donors weighing less than or equal to 10 kg into standard weight adult recipients: Transplantation, 2010; 90(5); 518-22
24. Yuan Q, Zhang L, Wang L, Outcome of a solitary kidney transplant into adult recipients from pediatric donors after controlled circulatory death: Exp Clin Transplant, 2011; 9(3); 165-69
25. Friedersdorff F, Fuller TF, Werthemann P, Cash H, Outcome of single pediatric deceased donor renal transplantation to adult kidney transplant recipients: Urol Int, 2014; 92(3); 323-27
26. Mohanka R, Basu A, Shapiro R, Kayler LK, Single versus en bloc kidney transplantation from pediatric donors less than or equal to 15 kg: Transplantation, 2008; 86(2); 264-68
27. Bretan PN, Friese C, Goldstein RB, Immunologic and patient selection strategies for successful utilization of less than 15 kg pediatric donor kidneys – long term experiences with 40 transplants: Transplantation, 1997; 63(2); 233-37
28. Shin HS, Grgic I, Chandraker A, Novel targets of immunosuppression in transplantation: Clin Lab Med, 2019; 39(1); 157-69
29. Jouve T, Rostaing L, Malvezzi P, New formulations of tacrolimus and prevention of acute and chronic rejections in adult kidney-transplant recipients: Expert Opin Drug Saf, 2017; 16(7); 845-55
30. Zhang R, Paramesh A, Florman S, Long-term outcome of adults who undergo transplantation with single pediatric kidneys: How young is too young?: Clin J Am Soc Nep, 2009; 4(9); 1500-6
31. Hara M, Kingsley C, Niimi M, IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo: J Immunol, 2001; 166(6); 3789-96
32. Graca L, Cobbold SP, Waldmann H, Identification of regulatory T cells in tolerated allografts: J Exp Med, 2002; 195(12); 1641-46
33. Li Y, Shi Y, Huang Z, CNI induced Th17/Treg imbalance and susceptibility to renal dysfunction in renal transplantation: Int Immunopharmacol, 2011; 11(12); 2033-38
34. Andreola G, Chittenden M, Shaffer J, Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation: Am J Transplant, 2011; 11(6); 1236-47
35. Dujardin A, Lorent M, Foucher Y, Time-dependent lymphocyte count after transplantation is associated with higher risk of graft failure and death: Kidney Int, 2021; 99(5); 1189-201
36. Wu Z, Bensinger SJ, Zhang J, Homeostatic proliferation is a barrier to transplantation tolerance: Nat Med, 2004; 10(1); 87-92
37. Sijpkens YW, Doxiadis II, Mallat MJ, Early versus late acute rejection episodes in renal transplantation: Transplantation, 2003; 75(2); 204-8
38. Vaziri ND, Pahl MV, Crum A, Norris K, Effect of uremia on structure and function of immune system: J Ren Nutr, 2012; 22(1); 149-56
39. Jiang Y, Song T, Qiu Y, Outcomes of single kidney transplantation from pediatric donors: A single-center experience: Pediatr Transplant, 2018; 22(5); e13196
40. Suneja M, Kuppachi S, Katz D, Hunsicker L, Small split pediatric kidneys to expand the donor pool: An analysis of Scientific Registry of Transplant Recipients (SRTR) data: Transplantation, 2019; 103(12); 2549-57
41. Sureshkumar KK, Reddy CS, Nghiem DD, Superiority of pediatric en bloc renal allografts over living donor kidneys: A long-term functional study: Transplantation, 2006; 82(3); 348-53
42. Nghiem DD, Hsia S, Schlosser JD, Growth and function of en bloc infant kidney transplants: A preliminary study: J Urol, 1995; 153(2); 326-29
43. Ahmed M, Venkataraman R, Logar AJ, Quantitation of immunosuppression by tacrolimus using flow cytometric analysis of interleukin-2 and interferon-gamma inhibition in CD8(−) and CD8(+) peripheral blood T cells: Ther Drug Monit, 2001; 23(4); 354-62
Figures
 Figure 1. Flow cytometry gating strategy. The lymphocyte population was selected based on FSC/SSC scatters: (a) CD4+ lymphocytes dot plot; (b) CD127 vs CD25 dot plot of CD4+ lymphocytes. D0: the day before KTx, D1: the first day after KTx, W1: the first-week after KTx, W2: the second-week after KTx, M1: the first month after KTx, M3: the third month after KTx, M6: the sixth month after KTx. Group C: 2 individual kidney recipients with stable renal function for 2 years after kidney transplantation. Group D: 2 healthy individuals.
Figure 1. Flow cytometry gating strategy. The lymphocyte population was selected based on FSC/SSC scatters: (a) CD4+ lymphocytes dot plot; (b) CD127 vs CD25 dot plot of CD4+ lymphocytes. D0: the day before KTx, D1: the first day after KTx, W1: the first-week after KTx, W2: the second-week after KTx, M1: the first month after KTx, M3: the third month after KTx, M6: the sixth month after KTx. Group C: 2 individual kidney recipients with stable renal function for 2 years after kidney transplantation. Group D: 2 healthy individuals. Figure 2. Dynamic changes of Tregs-to-CD4+ T cells ratio.
Figure 2. Dynamic changes of Tregs-to-CD4+ T cells ratio. Figure 3. The Tregs-to-CD4+ T cells ratio of groups C and D.
Figure 3. The Tregs-to-CD4+ T cells ratio of groups C and D. Figure 4. Dynamic changes of lymphocyte count.
Figure 4. Dynamic changes of lymphocyte count. Figure 5. The Tregs/CD4+ T cells ratio at various time point between kidney recipients from pediatric and adult donors.
Figure 5. The Tregs/CD4+ T cells ratio at various time point between kidney recipients from pediatric and adult donors. Figure 6. The level of Tregs/CD4+ T cell in infection and infection recovery.
Figure 6. The level of Tregs/CD4+ T cell in infection and infection recovery. Tables
 Table 1. Factors positively and negatively correlated with graft function.
Table 1. Factors positively and negatively correlated with graft function. Table 2. Factors associated with estimated glomerular filtration rate (correlation coefficient, p value).
Table 2. Factors associated with estimated glomerular filtration rate (correlation coefficient, p value). Table 3. Tregs/CD4+ T cells ratios and lymphocyte counts in median (Q25, Q75).
Table 3. Tregs/CD4+ T cells ratios and lymphocyte counts in median (Q25, Q75). Table 4. Demographic characteristics of transplant recipients and donors.
Table 4. Demographic characteristics of transplant recipients and donors. Table 5. Detailed eGFRs, creatinine levels and BUN amounts at various time points.
Table 5. Detailed eGFRs, creatinine levels and BUN amounts at various time points. Table 6. Tregs/CD4+ T cells ratios in the pediatric and adult donor groups in median (Q25, Q75).
Table 6. Tregs/CD4+ T cells ratios in the pediatric and adult donor groups in median (Q25, Q75). Table 1. Factors positively and negatively correlated with graft function.
Table 1. Factors positively and negatively correlated with graft function. Table 2. Factors associated with estimated glomerular filtration rate (correlation coefficient, p value).
Table 2. Factors associated with estimated glomerular filtration rate (correlation coefficient, p value). Table 3. Tregs/CD4+ T cells ratios and lymphocyte counts in median (Q25, Q75).
Table 3. Tregs/CD4+ T cells ratios and lymphocyte counts in median (Q25, Q75). Table 4. Demographic characteristics of transplant recipients and donors.
Table 4. Demographic characteristics of transplant recipients and donors. Table 5. Detailed eGFRs, creatinine levels and BUN amounts at various time points.
Table 5. Detailed eGFRs, creatinine levels and BUN amounts at various time points. Table 6. Tregs/CD4+ T cells ratios in the pediatric and adult donor groups in median (Q25, Q75).
Table 6. Tregs/CD4+ T cells ratios in the pediatric and adult donor groups in median (Q25, Q75). In Press
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








