19 March 2024: Original Paper
Long-Term Outcomes with Prolonged-Release Tacrolimus in Kidney Transplantation: A Retrospective Real-World Data Analysis
Wilfried Gwinner1ABDE*, Swapneel Anaokar2ACDE, Martin Blogg2ACDE, Birgit Hermann3ACDE, Carola del Pilar Repetur2CDE, Mario Schiffer4ABDEDOI: 10.12659/AOT.942167
Ann Transplant 2024; 29:e942167
Abstract
BACKGROUND: Long-term real-world outcomes data for kidney transplant recipients (KTRs) converting from immediate-release tacrolimus (IRT) to prolonged-release tacrolimus (PRT) are limited.
MATERIAL AND METHODS: A retrospective, non-interventional review of adult KTRs treated with PRT for ≥1 month was conducted in Germany. Data were extracted from time of transplant (2008-2014) to 2018. Primary composite endpoints (graft loss, biopsy-confirmed acute rejection, graft dysfunction) and secondary endpoints (all-cause mortality, kidney function course, and tacrolimus dose/trough levels) were analyzed for sub-cohorts: de novo PRT, early conversion from IRT (within 6 months post-transplant), and late conversion (7 months to 3 years).
RESULTS: Analysis included 163 patients (101 de novo, 12 early converters, and 50 late converters). The overall Kaplan-Meier estimate of freedom from efficacy failure through 5 years was 0.537, (95% confidence interval (CI) 0.455-0.612) (de novo: 0.512 [0.407-0.608]; early converters: 0.500 [0.208-0.736]; late converters: 0.594 [0.443-0.717]). The overall survival rate was 0.925 (95% CI 0.872-0.957) (de novo: 0.900 [0.823-0.945]; early converters: 0.917 [0.539-0.988]; late converters: 0.977 [0.846-0.997]). During follow-up, there was a gradual reduction in tacrolimus dose and trough levels; kidney function remained stable in all cohorts. Multivariable analysis found re-transplantation, organ donor quality, best estimated glomerular filtration rate 8-12 weeks after transplant, and treatment center (between-center differences in age, sex, donor status/quality) were significantly associated with efficacy failure.
CONCLUSIONS: There was no difference in long-term survival profiles between KTRs who received PRT de novo vs those who converted from IRT, with 5-year survival remaining high in both groups.
Keywords: Immunosuppression Therapy, Tacrolimus, Kidney Transplantation, Retrospective Studies, clinical study
Introduction
Despite advances in immunosuppressive therapy and improvements in 1-year transplant survival rates over several decades [1,2], long-term survival after kidney transplantation remains challenging, with data from the US and Europe suggesting that 10-year graft survival is less than 60% [3].
Tacrolimus is the mainstay of post-kidney transplant immunosuppression, commonly used with other immunosuppressants as dual- or triple-drug regimens to prevent acute kidney rejection [4,5]. While short-term patient and graft survival rates after kidney transplant are high, longer-term graft survival may be jeopardized by nonadherence to immunosuppressive regimens [5,6]. A prospective study following kidney transplant recipients (KTRs) for 18 years found nonadherence to be a major cause of graft loss, with most nonadherence occurring 3 years or more after transplantation [6]. For tacrolimus, which has a narrow therapeutic index, adherence and maintenance of stable blood levels are important [5]. Variability in systemic tacrolimus exposure is a risk factor for graft loss in the early and late post-transplantation period [7,8] and reducing intra-patient variability in tacrolimus exposure has been shown to improve graft survival [9,10].
In addition to the traditional twice-daily immediate-release tacrolimus (IRT) formulation, a once-daily prolonged-release tacrolimus (PRT) formulation has been developed to provide a more consistent level of total tacrolimus exposure and more convenient dosing regimen [5,11–13]. Benefits of PRT over IRT include improved patient adherence [14] and reduced intra-patient variability in tacrolimus exposure [11,12], both of which are determinants of long-term graft outcome, with no difference in exposure [7,8,15–19]; however, there could be some formulation and pharmacokinetic differences between PRT medications. Additionally, the benefit of switching to PRT may be impacted by the exact formulation used; recent data showed differences in intestinal absorption between 2 formulations of PRT, leading to differences in variability of exposure [20].
The efficacy and safety of PRT is well established with respect to short-term outcomes, in both de novo KTRs and those switching from IRT [21]. Longer-term outcomes with PRT are less well characterized, but the limited trial data that are available are encouraging [22,23]. A 5-year, non-interventional, prospective follow-up study of KTRs randomized to de novo PRT in the Phase IV, open-label, ADHERE (1-year) study revealed patient and graft survival rates of 91.9% and 89.3%, respectively [22]. Similar 5-year patient and graft survival rates (94.4% and 88.1%) were seen in a follow-up of KTRs receiving de novo PRT in the Phase IV, open-label, 24-week ADVANCE trial [24]. In a 5-year open-label, single-center, prospective study of KTRs in Japan, the patient survival rate with de novo PRT was 96.7% at 5 years after transplantation, with 6.8% of patients having graft loss and a 5-year Kaplan-Meier (KM) estimated rejection rate of 26.9% [23]. In the ADMIRAD follow-up study, a retrospective non-interventional chart review of KTRs treated with PRT following conversion from IRT, the KM survival rate without efficacy failure (defined as transplantation, nephrectomy, death or return to dialysis) from randomization to year 5 was 74.1%, and overall patient survival was 98.1% and 88.0% at 5 and 10 years after transplant, respectively [25].
There is a need to further characterize long-term clinical outcomes with PRT in both de novo patients and those converting from IRT. The aim of this retrospective study was to assess the long-term outcomes of PRT-based immunosuppressive therapy in de novo KTRs or those converting from IRT to PRT in a real-world clinical practice setting.
Material and Methods
STUDY DESIGN AND POPULATION:
This was a retrospective, non-interventional, observational chart review conducted at 2 kidney transplant centers in Germany: Universitätsklinikum Erlangen (Center 1) and Medizinische Hochschule Hannover (Center 2).
Patients were included if they were ≥18 years of age, had received a kidney transplant between 2008 and 2014 from a deceased or living donor, and had received PRT-based therapy (Advagraf®, Astellas Pharma Europe BV, Leiden, the Netherlands) for ≥1 month either de novo or after conversion from IRT (Prograf®, Astellas Pharma Europe BV, Leiden, the Netherlands). PRT-based therapy was defined as PRT at a strength of 0.5 mg, 1 mg, 3 mg, and 5 mg; capsules were administered orally once daily, either de novo or after conversion from IRT. Patients were excluded if they had contraindications for tacrolimus treatment, had received IRT >3 years prior to conversion to PRT, had switched from a non-tacrolimus-based immunosuppressive regimen, or had received a multi-organ transplant. Patients could have received a kidney from a deceased or living donor. Minimum thresholds for inclusion based on estimated glomerular filtration rate (eGFR) at baseline were not defined.
The study was conducted in compliance with national and European Union requirements for ensuring the rights of participants in non-interventional studies and followed the regulations of the Declaration of Helsinki and the Declaration of Istanbul. Approval was obtained from the Institutional Ethics Commission at both centers. Patients who were alive at the time of data extraction provided written informed consent. The requirement for informed consent was waived for patients who had died before the start of the study. The reporting of this study conforms to the STROBE statement [26].
DATA EXTRACTION:
Data were sourced from the medical charts of transplant recipients at Center 1 and Center 2. For each eligible patient, retrospective data from time of transplant (2008–2014) until the date of the last follow-up visit prior to December 31, 2018 (maximum 11-year period) were extracted from existing medical records by center personnel using an electronic case report form. Data were recorded as part of routine care and no specific patient visits were required as part of the study. Collected data included: date of PRT initiation or conversion from IRT; reason for conversion to PRT from IRT; drug dose; tacrolimus blood trough level; date of graft loss and type of graft loss (re-transplantation, death, or first day of dialysis); date of biopsy-confirmed acute rejection (BCAR); date of occurrence of graft dysfunction, defined as eGFR of <40 mL/min/1.73 m2 according to the modification of diet in renal disease-4 (MDRD-4) formula; renal parameters from laboratory assessment, including serum creatinine, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and baseline eGFR, defined as best eGFR 8–12 weeks post-transplant (MDRD-4); and cause of patient death. Missing data were requested from the treating nephrologists by center personnel.
ENDPOINTS:
The primary composite endpoint of efficacy failure was the earliest occurrence of any of the following: (1) graft loss, including re-transplantation, death or dialysis; (2) BCAR, determined by the investigator and regarded as acute antibody-mediated rejection (grade I, II or III) or acute T-cell-mediated rejection (grade IA, IB, IIA, IIB or III), according to Banff criteria valid at the time of biopsy [27]; or (3) graft dysfunction, defined previously. Patients who never had eGFR ≥40 mL/min/1.73 m2 were considered to have graft dysfunction on the day of first occurrence of eGFR <40 mL/min/1.73 m2, which could be on day 1.
Secondary endpoints included all-cause mortality, kidney function over time, and prescribed tacrolimus dosing and trough levels. In addition, exploratory analyses assessed independent risk factors for impact on the composite endpoint.
SUB-GROUP AND POST HOC ANALYSES:
Primary analyses of endpoints were conducted for the full analysis cohort (FAC), comprising all eligible patients who received ≥1 month of continuous PRT following kidney transplantation, and 3 sub-groups as follows:
Post hoc analyses included comparison of the primary endpoint (and individual components of the composite) between Center 1 and Center 2 and the association of further variables with the primary endpoint including; cohort (de novo vs combined conversion [early plus late conversion sub-groups]), baseline kidney function (8–12 weeks after transplantation), and the impact on kidney function in patients who had converted from IRT (combined conversion sub-group) versus the de novo PRT sub-group. Post-transplant prescribed tacrolimus doses and trough levels from time of transplant were also assessed in the FAC and combined conversion cohorts.
STATISTICAL ANALYSIS:
Continuous data were reported as mean (standard deviation [SD]), median (lower quartile [Q1], upper quartile [Q3]). Categorical data were reported as frequencies and percentages. Patients with missing data were not excluded, and each variable was summarized based on the available data. Primary and secondary endpoint and post hoc analyses were conducted on all study cohorts.
Analysis of the primary composite endpoint provided KM estimates and 95% confidence intervals (95% CIs) of the time from transplant to the first incidence of the components of the endpoint, using Greenwood’s formula to estimate the standard error of survival. Statistical significance was determined using log-rank testing of all events captured over the follow-up period. KM estimates (with 95% CI, calculated as defined above) of the composite endpoint at 5 years (the primary objective) and for each year of follow-up were calculated, as well as the estimated median time to composite endpoint (with 95% CI). Patients who were lost to follow-up or were alive at the end of the study without occurrence of the endpoint were censored on the day of the last available follow-up visit. For the secondary endpoints, all-cause mortality was analyzed using KM methods as per the primary composite endpoint. Other secondary endpoints were analyzed using descriptive statistics, with baseline measurements performed on the date of transplantation. Post hoc analyses compared the KM estimates of freedom from efficacy failure between the de novo and combined conversion (early plus late) cohorts and the primary composite endpoint by eGFR at baseline (8–12 weeks post-transplant) using fixed thresholds for eGFR categories (<30 mL/min/1.73 m2, ≥30–<45 mL/min/1.73 m2, ≥45–<60 mL/min/1.73 m2, and ≥60 mL/min/1.73 m2). Descriptive graphs plotting means and standard errors of eGFR over time from time of transplant were plotted for the de novo and combined conversion cohort. Patients with graft loss were included in the analysis of subsequent visits and assigned an eGFR of 0. Sensitivity analyses, conducted to test the robustness of the primary composite endpoint, included KM estimates of the individual components of the composite endpoint and patients with and without BCAR or kidney dysfunction prior to conversion.
In the exploratory analysis, independent risk factors were modeled using a Cox regression model adjusting for potential confounders. Univariate models were used to determine the variables associated with PRT effectiveness and a final multivariable model was developed to quantify the contribution of each variable to the between-patient variation in outcome. The models used eGFR according to the MDRD-4 formula. The Cox multivariable models (exploratory and post hoc analyses) used forward selection (
Results
PRIMARY COMPOSITE ENDPOINT:
Overall, 76 (46.6%) patients had a primary composite endpoint event (de novo 49 [48.5%]; early converters 6 [50.0%]; late converters 21 [42.0%]), and 87 (53.4%) patients were censored. Post hoc analysis revealed 60.5% of patients had a primary composite endpoint event at Center 1 vs 32.9% at Center 2 (Table 3).
KM estimates of time from transplant to first primary composite endpoint event are presented in Figure 2. The KM estimate of median time to primary endpoint event was 7.11 years (95% CI 3.87-not estimable [NE]). There was no clear difference in the 5-year time-to-event profile between the cohorts (log-rank test P=0.744) and the KM estimate of freedom from efficacy failure through 5 years was 0.537 (95% CI 0.455–0.612) (Table 4).
SENSITIVITY ANALYSES OF THE PRIMARY COMPOSITE ENDPOINT:
The KM estimates of individual components of the primary composite endpoint are presented only as a sensitivity analysis owing to potential bias due to the competing risks of occurrence of the other primary endpoint components. When each component was analyzed individually, there was no major difference between the de novo, early conversion, and late conversion cohorts (Table 5). The survival rate without experiencing graft loss in the FAC was 0.862 (95% CI 0.794–0.909) at 5 years. When combining early and late conversion cohorts into a single ‘conversion’ cohort in the post hoc analysis, there was also no significant difference between the cohorts (log-rank test P=0.603; Figure 3). The KM estimate of freedom from efficacy failure through 5 years was 0.512 (95% CI 0.407–0.608) in the de novo PRT cohort and 0.576 (95% CI 0.442–0.689) in the combined (early plus late) conversion cohort. The crude incidence of primary endpoint event by the end of the study (date of the last available follow-up visit) was 48.5% (49/101) in the de novo PRT cohort and 43.5% (27/62) in the combined conversion cohort.
There were 22 patients in the primary analysis who had graft dysfunction or BCAR prior to conversion to PRT (17 of whom were included in the late conversion cohort). Sensitivity analysis of the primary composite endpoint for the remaining 141 patients after exclusion of these patients revealed a KM estimate of freedom from efficacy failure through 5 years of 0.622 (95% CI 0.533–0.698).
SURVIVAL:
Overall, 18 (11.0%) patients died over the maximum post-transplant study period (11 years). The KM estimate of survival rate at 5 years was 0.925 (95% CI 0.872–0.957) in the FAC and remained high in all cohorts: (0.900 (95% CI 0.823–0.945) for the de novo cohort, 0.917 (95% CI 0.539–0.988) for the early conversion cohort, and 0.977 (95% CI 0.846–0.997) for the late conversion cohort (Table 6).
IMPACT OF INDEPENDENT VARIABLES ON COMPOSITE PRIMARY ENDPOINT:
All variables with P<0.2 in the univariate analysis (results shown in Table 7) were considered for multivariable model entry. The multivariable Cox proportional hazards analysis revealed variables significantly associated with the primary composite endpoint (P≤0.001) including: number of previous transplants (1 vs 0; hazard ratio [HR] 6.592; 95% CI 2.331–18.639); donor quality (ECD vs standard criteria donor [SCD] status: HR 2.931; 95% CI 1.554–5.526); baseline eGFR (best eGFR 8–12 weeks post-transplant, time-dependent variable: HR 0.933; 95% CI 0.894–0.974); as well as study site (Center 2 vs Center 1: HR 0.196; 95% CI 0.078–0.494) (Table 7).
TACROLIMUS DOSE AND TROUGH LEVELS:
Tacrolimus doses prescribed over time were captured in a descriptive plot stratified by conversion cohorts (Figure 4A), which shows that mean prescribed doses were similar for the de novo and late conversion cohorts throughout the follow-up period. Mean doses in the early conversion cohort, which had considerably fewer patients (n=12 vs n=101 and n=50, in the de novo and late conversion cohorts, respectively) were comparatively lower. The tacrolimus dose curves in all 3 cohorts followed a downward gradient, indicating a gradual tapering of dosage over time before the dose became stable. Similarly, in all 3 cohorts, tacrolimus trough levels gradually decreased over time before whole blood tacrolimus trough levels reached a stable level (Figure 4B). At baseline, the mean tacrolimus trough levels ranged from 10–12 ng/mL in all cohorts. The mean levels in all cohorts remained generally similar throughout the duration of follow-up.
KIDNEY FUNCTION:
In the FAC, mean serum creatinine was 1.67 mg/mL at 8–12 weeks post-transplant. While all laboratory assessments were similar in the de novo and late conversion cohorts, metrics differed in the early conversion cohort, including decreased serum creatinine and tacrolimus trough level and increased eGFR and CKD-EPI (Table 8).
When the early conversion cohort was combined with the late conversion cohort, mean eGFR values were similar to the de novo cohort and remained stable through 5 years and beyond (Figure 5).
Post hoc analysis of the primary composite endpoint by eGFR at baseline (8–12 weeks post-transplant) showed that the lowest category of eGFR (<30 mL/min/1.73 m2) was associated with the highest cumulative proportion of patients reporting primary composite endpoint event at 5 years post-transplant (crude incidence 75.0% vs 31.0–50.0% for eGFR categories ≥30 mL/min/1.73 m2). The KM estimates of freedom from efficacy failure increased across the eGFR categories, with the lowest event-free rate through 5 years of 0.208 (95% CI 0.044–0.455) in the eGFR <30 mL/min/1.73 m2 category and highest rate of 0.690 (95% CI 0.488–0.825) in the eGFR ≥60 mL/min/1.73 m2 category (Table 9).
Discussion
This retrospective real-world analysis of KTRs from 2 study centers in Germany was conducted to address the evidence gap in long-term outcomes with PRT. Data were collected from transplant recipients receiving PRT who were also stratified into 3 sub-cohorts depending on the time from kidney transplant to initiation of PRT: within the first month (de novo), within 6 months (early conversion from IRT), or within 3 years (late conversion from IRT). In this way, outcomes could be explored across patients with a broad spectrum of transplant-related medical histories (eg, renal impairment or rejection events prior to initiating PRT). The maximum period a patient could be followed post-transplantion was 11 years and median time between initiating PRT to discontinuation, censoring, or loss-to-follow-up was ~4.6 years.
For the entire population, the KM estimates of freedom from efficacy failure (primary composite endpoint) and overall survival through 5 years were 0.537 (95% CI 0.455–0.612) and 0.925 (95% CI 0.872–0.957), respectively, and there was no difference in 5-year time-to-event profiles between de novo and conversion patients. The KM estimate of median time to primary endpoint event was 7.11 years (95% CI 3.87-NE). Previous studies of IRT to PRT conversion over shorter-term observation periods (1–2 years) have presented similar findings, with no marked differences observed between early and late stages of conversion [28,29]. Further, a meta-analysis exploring outcomes across 9 randomized controlled trials comparing IRT or PRT in de novo KTRs found similar between-group rates of acute rejection, graft failure, death, and adverse events after 12 months, suggesting comparable efficacy and safety of these 2 regimens [30]. However, the current retrospective analyses aimed to assess the longer-term outcomes in a real-world setting in kidney transplant patients in Germany. Three previous studies have also reported 5-year patient and graft survival with PRT, but not for patients converting from IRT to PRT or using a stringent composite primary endpoint [22,23]. The first study, an open-label, prospective, observational, multicenter, post-marketing surveillance study in Japan [23], reported higher 5-year patient survival and graft survival rates (96.7% and 93.2%, respectively) than those reported in the current study (92.5% and 86.2% [graft survival from sensitivity analysis]). However, 94% of patients in the Japanese study received a kidney from a living donor (compared with 34% in the current study), 59% of recipients were male (compared with 72% in the current study), and only 7% were aged ≥65 years (compared with 17% in the current study). Post hoc analysis comparing the demographic characteristics and outcomes between Center 1 and Center 2 in our study, as well as previous reports [31,32], show that these factors can markedly influence outcomes. Incidence of graft loss was more than 3 times greater at Center 1 versus Center 2 (25% vs 7%); of which, Center 1 had a recipient population with a smaller proportion of living donors (29% vs 43%) and a larger proportion with ECD status (44% vs 11%), more patients were aged ≥65 years (25% vs 10%), and with comorbidities (22% vs 7%). These results suggest that there may be further variables affecting outcomes between the 2 centers that were not considered in the multivariable analysis, such as donor serum creatinine. The second study, a prospective multicenter 5-year follow-up of the ADHERE study in a European population with a demographic profile that was similar to the current study in terms of age, sex, and donor type, demonstrated patient survival (91.9%) and graft survival (89.3%) rates with PRT that were similar to those observed in the current study [22]. More recent data from the ADVANCE follow-up trial, a 5-year follow-up of KTRs receiving de novo PRT, reported higher patient survival rates (94.4%) versus the present study, but lower graft survival rates (88.1%) [24]. However, the prevalence of key factors that can influence outcomes [31,32] varied versus the present study; at most, 14.6% of patients received a kidney from a living donor in each study arm, and 65.1% of recipients were male. There were also some differences in the definition of endpoints versus the present study.
Additionally, the ADMIRAD retrospective chart analysis study explored long-term outcomes up to 10 years for KTRs converting from IRT to PRT [25]. In contrast to the present study, results for 5-year follow-up from ADMIRAD showed that the KM survival rate without efficacy failure (defined as transplantation, nephrectomy, death or return to dialysis) in ADMIRAD was 74.1% for the PRT group and 66.7% for the IRT group and remained higher for PRT throughout follow-up (
In both the de novo and conversion cohorts in the present study, there was a gradual reduction of mean tacrolimus daily dose and trough levels before doses, and trough levels became more stable around 18 months to 2 years post-transplantation. Nevertheless, stable kidney function was observed in the full patient cohort and sub-cohorts of de novo and conversion PRT throughout the entire period. This is consistent with previous observations where eGFR values were unaffected by reduced tacrolimus dose and trough levels after IRT to PRT conversion [33,34]. Multivariable analysis showed that donor quality, previous transplantation, and the eGFR value 8–12 weeks post-transplant were significantly associated with the composite endpoint for efficacy failure (
Key strengths of the current study include the long post-transplantation period over which patient data extraction could take place. However, the study also has several potential limitations: selection of patients from 2 clinical sites in Germany potentially introduces selection bias and may not be representative of the wider population of kidney transplant patients; only descriptive analyses were conducted, although this approach aligns with the lack of a comparator group; and adherence with immunosuppressive therapy was not assessed, despite being a known factor impacting long-term graft survival. There were also few patients in the early conversion cohort and the attrition rate in the late conversion cohort resulted in a low sample size by year 5, which limits the generalizability of findings for these data; one possibility for this attrition rate may be due to graft loss experienced by patients, and who were then no longer in the care of the transplant center (data censored). However, this graft loss would be included in the time to composite endpoint and time to graft loss analyses. Despite these limitations, the current study provides clinicians with real-world long-term information about the use of PRT in a diverse group of recipients.
Conclusions
In this retrospective study of KTRs treated with PRT in Germany, over 50% of patients had event-free survival through 5 years, and overall survival was over 90%. There was no difference in 5-year time-to-event profiles between de novo and conversion cohorts, and kidney function remained stable for both cohorts, with no new safety findings observed. These results support existing data demonstrating the effectiveness and safety of long-term PRT use in KTRs.
Figures
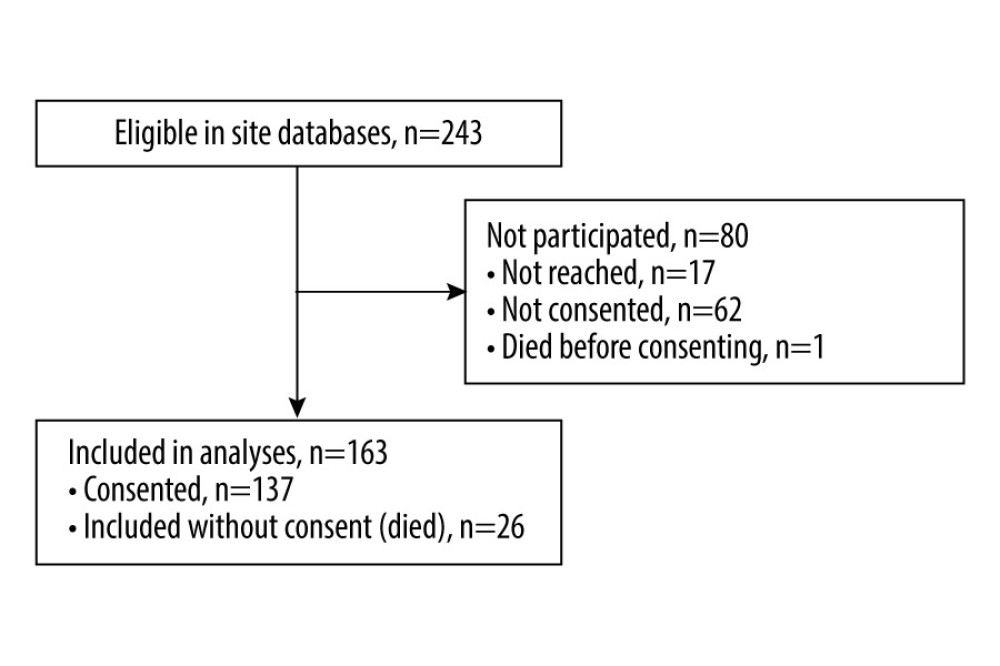 Figure 1. Patient flow through the study.
Figure 1. Patient flow through the study. 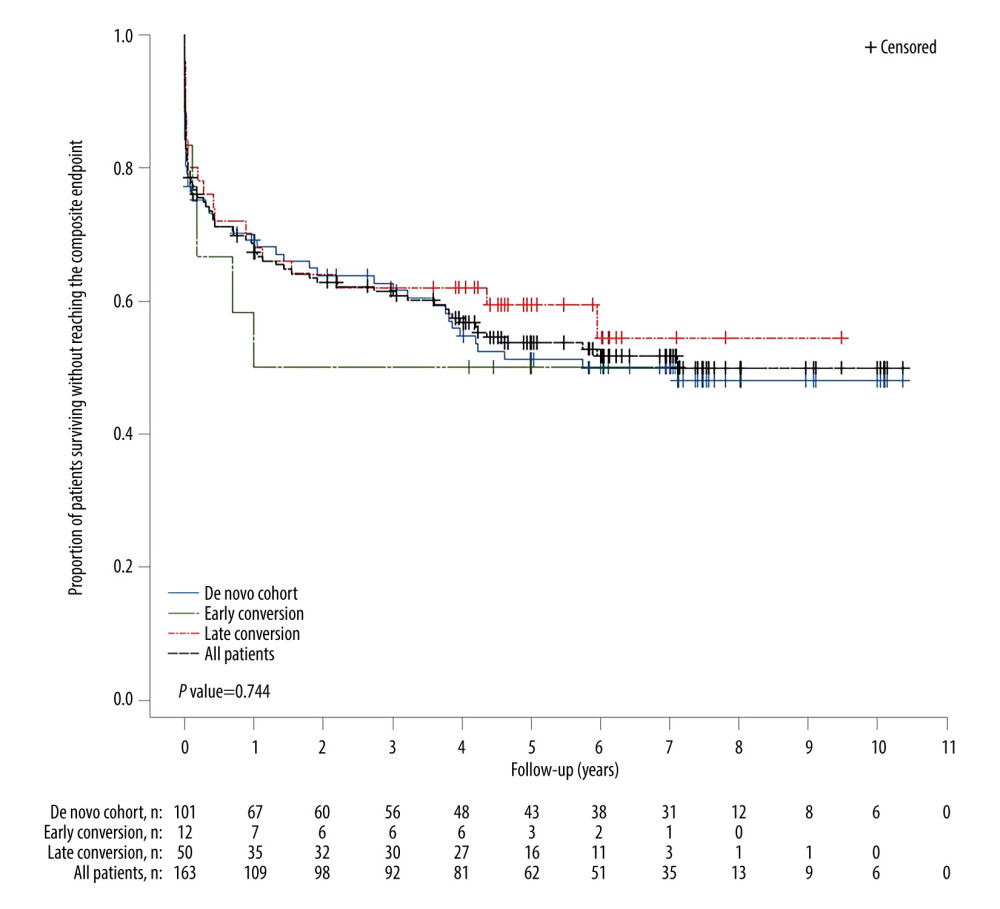 Figure 2. Kaplan-Meier plot of time from transplant to primary composite endpoint event in the full analysis cohort and the 3 tacrolimus conversion cohorts. P-value is based on time to event though 5 years.
Figure 2. Kaplan-Meier plot of time from transplant to primary composite endpoint event in the full analysis cohort and the 3 tacrolimus conversion cohorts. P-value is based on time to event though 5 years. 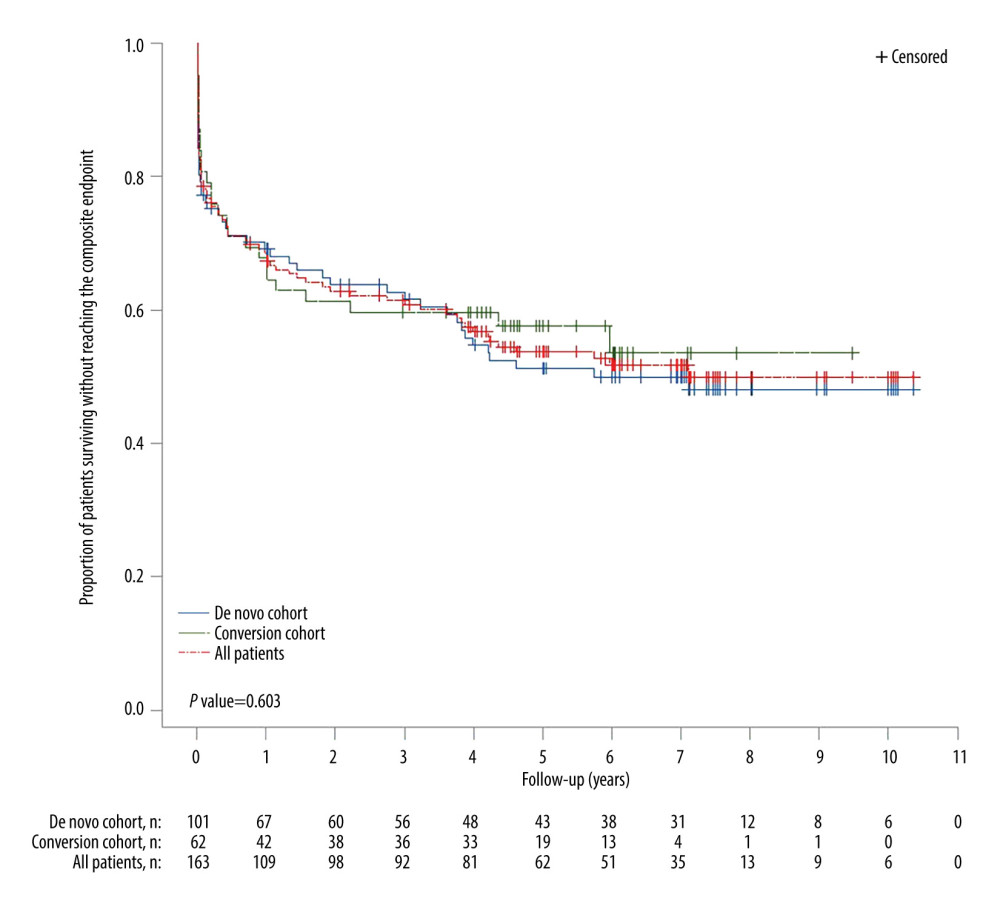 Figure 3. Kaplan-Meier plot of time from transplant to primary composite endpoint event in the full analysis cohort, de novo, and tacrolimus conversion (combined early and late conversion) cohorts. P-value is based on time to event through 5 years.
Figure 3. Kaplan-Meier plot of time from transplant to primary composite endpoint event in the full analysis cohort, de novo, and tacrolimus conversion (combined early and late conversion) cohorts. P-value is based on time to event through 5 years. 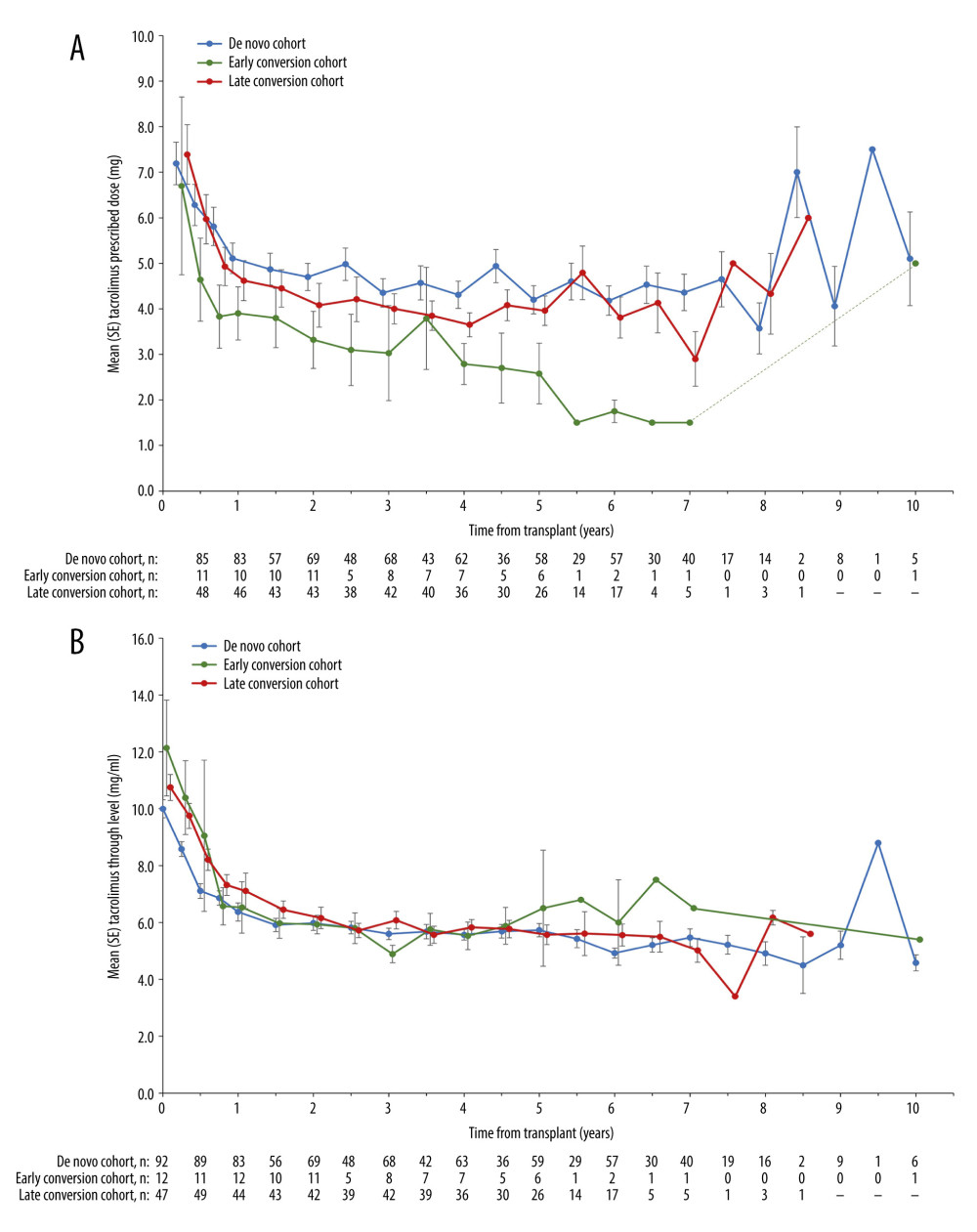 Figure 4. (A) Mean prescribed tacrolimus dosages from time of transplant, stratified by de novo, early- and late-conversion cohorts. Error bars denote standard error. (B) Mean tacrolimus trough levels from time of transplant, stratified by de novo, early- and late-conversion cohorts. Error bars denote standard error.
Figure 4. (A) Mean prescribed tacrolimus dosages from time of transplant, stratified by de novo, early- and late-conversion cohorts. Error bars denote standard error. (B) Mean tacrolimus trough levels from time of transplant, stratified by de novo, early- and late-conversion cohorts. Error bars denote standard error. 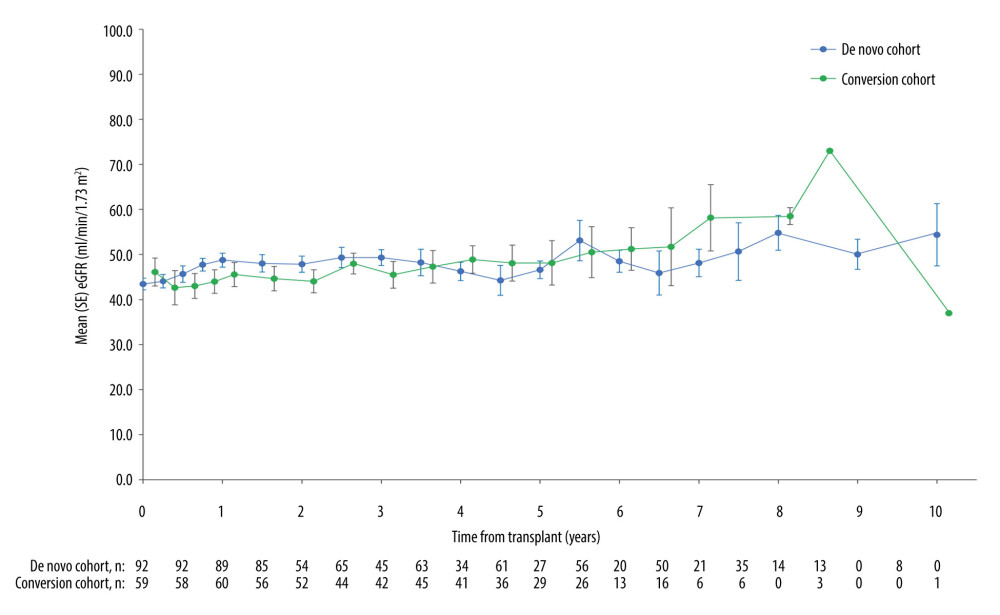 Figure 5. Mean (SE) eGFR values from time of transplant, stratified by de novo and combined conversion cohorts (early plus late conversion). Patients with graft loss were included in the analysis of subsequent visits and assigned an eGFR of 0. eGFR – estimated glomerular filtration rate; SE – standard error.
Figure 5. Mean (SE) eGFR values from time of transplant, stratified by de novo and combined conversion cohorts (early plus late conversion). Patients with graft loss were included in the analysis of subsequent visits and assigned an eGFR of 0. eGFR – estimated glomerular filtration rate; SE – standard error. Tables
Table 1. Baseline characteristics of patients.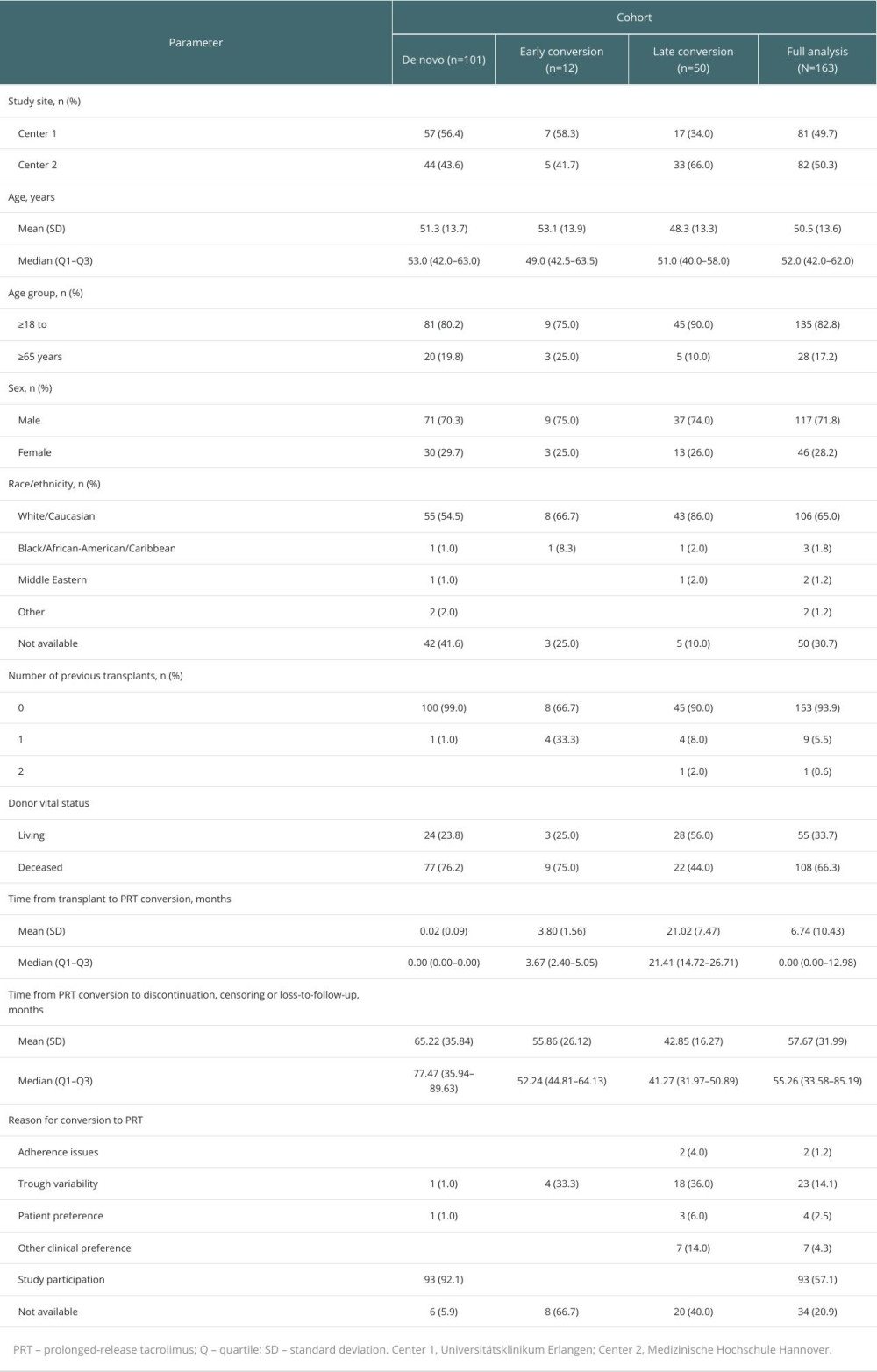 Table 2. Patient demographics per transplant center.
Table 2. Patient demographics per transplant center.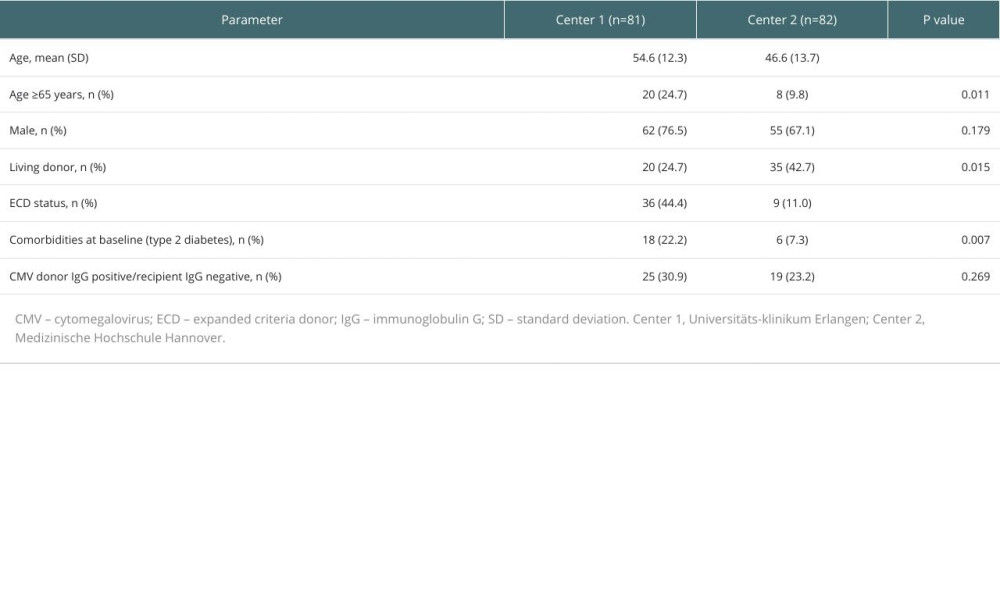 Table 3. Post hoc analysis of outcomes per transplant center.
Table 3. Post hoc analysis of outcomes per transplant center.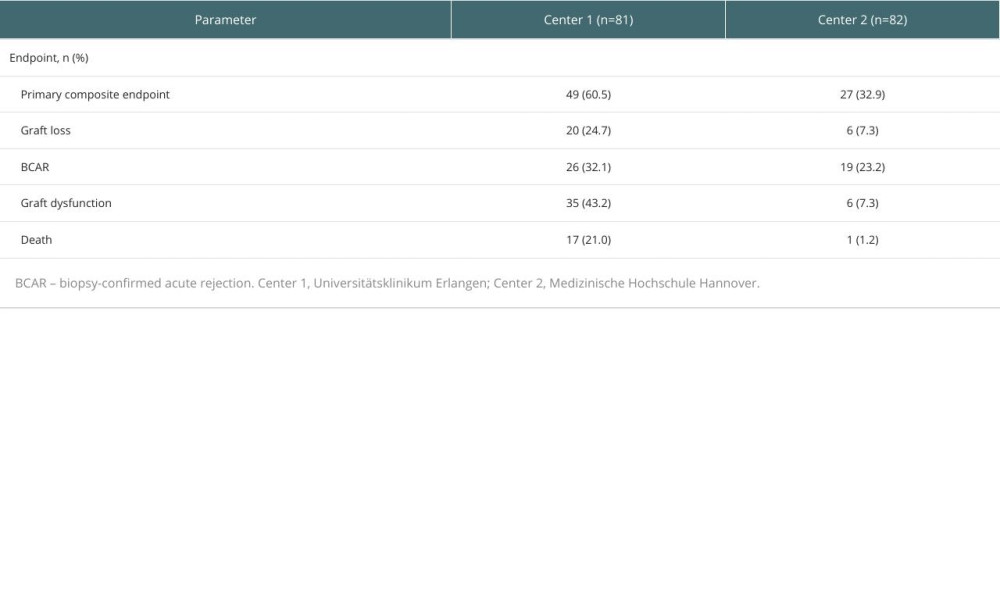 Table 4. Kaplan-Meier estimates of freedom from the primary composite endpoint stratified by population cohort (first 5 years post-transplant).
Table 4. Kaplan-Meier estimates of freedom from the primary composite endpoint stratified by population cohort (first 5 years post-transplant).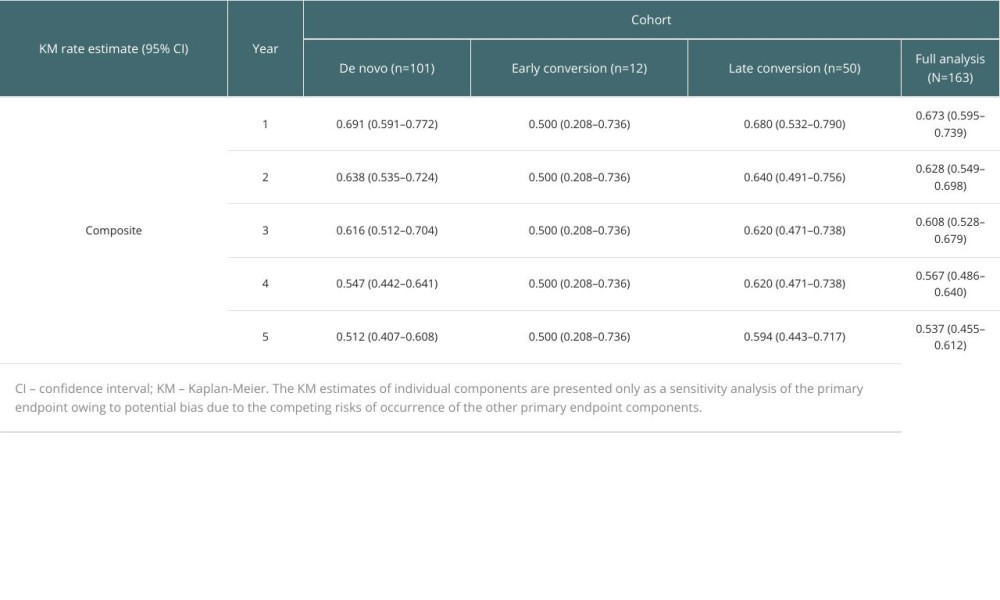 Table 5. Sensitivity analysis of primary endpoint: Kaplan-Meier estimates of the individual components of the composite endpoint stratified by population cohort (first 5 years post-transplant).
Table 5. Sensitivity analysis of primary endpoint: Kaplan-Meier estimates of the individual components of the composite endpoint stratified by population cohort (first 5 years post-transplant).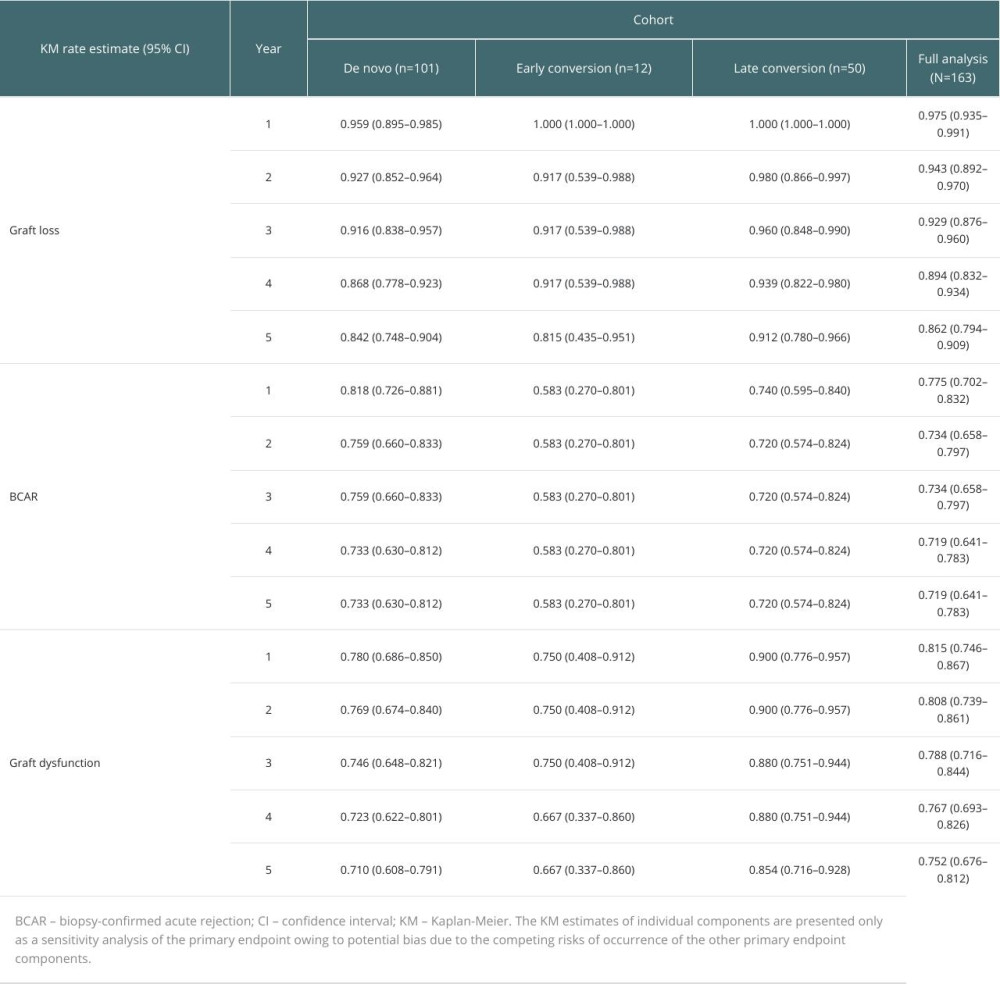 Table 6. Kaplan-Meier estimates of patient survival stratified by population cohort (first 5 years post-transplant).
Table 6. Kaplan-Meier estimates of patient survival stratified by population cohort (first 5 years post-transplant).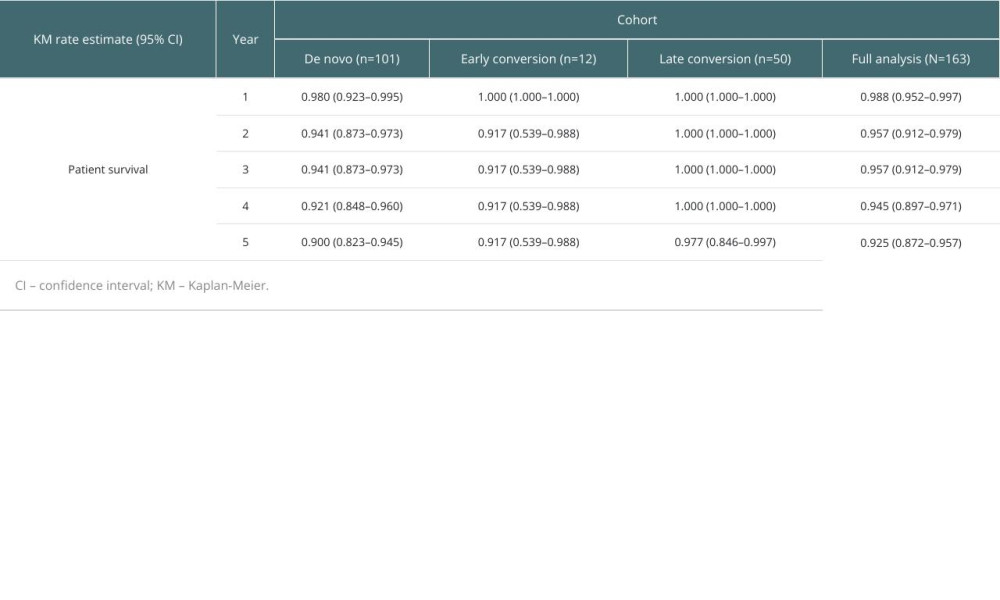 Table 7. Multivariable analysis of independent variables associated with the primary composite endpoint for all patients.
Table 7. Multivariable analysis of independent variables associated with the primary composite endpoint for all patients.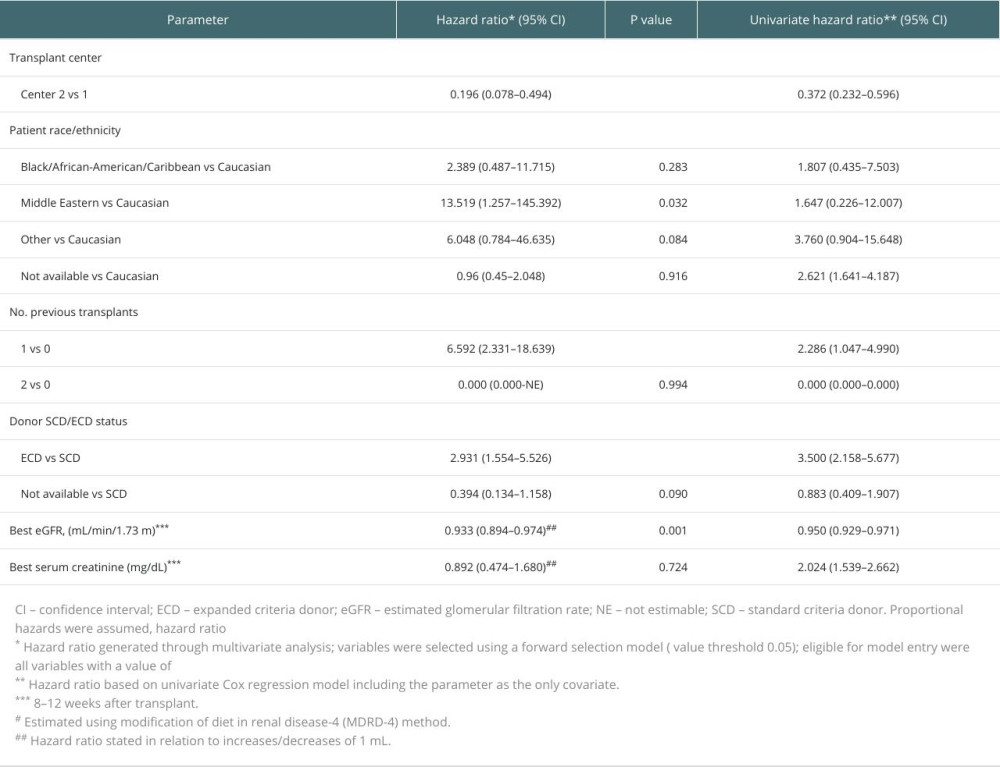 Table 8. Laboratory assessments at 8–12 weeks after transplant by population cohort.
Table 8. Laboratory assessments at 8–12 weeks after transplant by population cohort.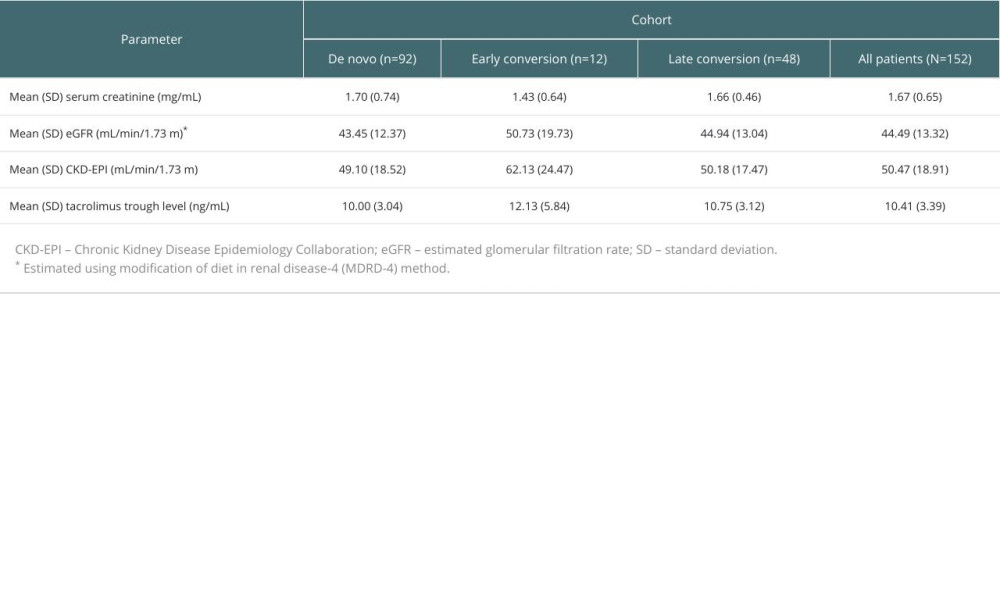 Table 9. Kaplan-Meier estimates of primary composite endpoint stratified by baseline eGFR.
Table 9. Kaplan-Meier estimates of primary composite endpoint stratified by baseline eGFR.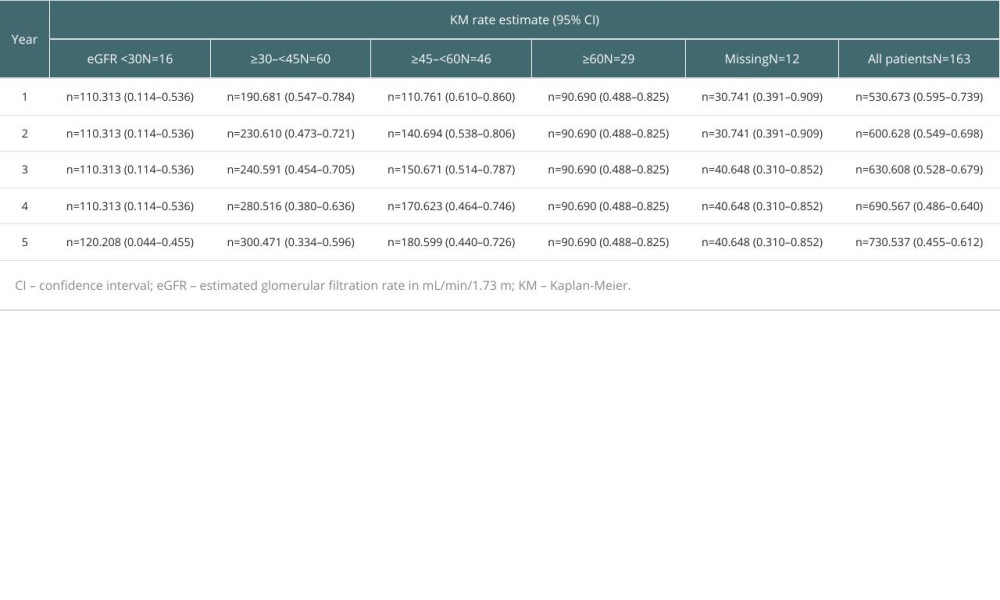
References
1. Lamb KE, Lodhi S, Meier-Kriesche HU, Long-term renal allograft survival in the United States: A critical reappraisal: Am J Transplant, 2011; 11(3); 450-62
2. Hart A, Smith JM, Skeans MA, OPTN/SRTR 2017 Annual Data Report: Kidney: Am J Transplant, 2019; 19; 19-123
3. Gondos A, Döhler B, Brenner H, Opelz G, Kidney graft survival in Europe and the United States: Strikingly different long-term outcomes: Transplantation, 2013; 95; 267-74
4. Hariharan S, Israni AK, Danovitch G, Long-term survival after kidney transplantation: N Engl J Med, 2021; 385(8); 729-43
5. Banas B, Krämer BK, Krüger B, Long-term kidney transplant outcomes: Role of prolonged-release tacrolimus: Transplant Proc, 2020; 52(1); 102-10
6. Gaynor JJ, Ciancio G, Guerra G, Graft failure due to noncompliance among 628 kidney transplant recipients with long-term follow-up: A single-center observational study: Transplantation, 2014; 97; 925-33
7. Borra LC, Roodnat JI, Kal JA, High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation: Nephrol Dial Transplant, 2010; 25; 2757-63
8. Sapir-Pichhadze R, Wang Y, Famure O, Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure: Kidney Int, 2014; 85(6); 1404-11
9. Rahamimov R, Tifti-Orbach H, Zingerman B, Reduction of exposure to tacrolimus trough level variability is associated with better graft survival after kidney transplantation: Eur J Clin Pharmacol, 2019; 75(7); 951-58
10. Gold A, Tönshoff B, Döhler B, Süsal C, Association of graft survival with tacrolimus exposure and late intra-patient tacrolimus variability in pediatric and young adult renal transplant recipients – an international CTS registry analysis: Transpl Int, 2020; 33(12); 1681-92
11. Wu MJ, Cheng CY, Chen CH, Lower variability of tacrolimus trough concentration after conversion from Prograf to Advagraf in stable kidney transplant recipients: Transplantation, 2011; 92; 648-52
12. Stifft F, Stolk LML, Undre N, van Hooff JP, Christiaans MHL, Lower variability in 24-hour exposure during once-daily compared to twice-daily tacrolimus formulation in kidney transplantation: Transplantation, 2014; 97; 775-80
13. Tanzi MG, Undre N, Keirns J, Pharmacokinetics of prolonged-release tacrolimus and implications for use in solid organ transplant recipients: Clin Transplant, 2016; 30; 901-11
14. Kuypers DRJ, Peeters PC, Sennesael JJ, Improved adherence to tacrolimus once-daily formulation in renal recipients: A randomized controlled trial using electronic monitoring: Transplantation, 2013; 95(2); 333-40
15. Sellarés J, de Freitas DG, Mengel M, Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence: Am J Transplant, 2012; 12(2); 388-99
16. Wiebe C, Gibson IW, Blydt-Hansen TD, Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody: Am J Transplant, 2015; 15(11); 2921-30
17. Rodrigo E, Segundo DS, Fernández-Fresnedo G, Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development: Transplantation, 2016; 100(11); 2479-85
18. Whalen HR, Glen JA, Harkins V, High intrapatient tacrolimus variability is associated with worse outcomes in renal transplantation using a low-dose tacrolimus immunosuppressive regime: Transplantation, 2017; 101(2); 430-36
19. Monchaud C, Woillard JB, Crépin S, Tacrolimus exposure before and after a switch from twice-daily immediate-release to once-daily prolonged release tacrolimus: The ENVARSWITCH study: Transpl Int, 2023; 36; 11366
20. Kolonko A, Słabiak-Błaż N, Pokora P, Intestinal permeability in patients early after kidney transplantation treated with two different formulations of once-daily tacrolimus: Int J Mol Sci, 2023; 24(9); 8344
21. Caillard S, Moulin B, Buron F: Transpl Int, 2016; 29; 860-69
22. Rummo O, Carmellini M, Kamar N, Long-term, prolonged-release tacrolimus-based immunosuppression in de novo kidney transplant recipients: 5-year prospective follow-up of the ADHERE study patients: Transpl Int, 2020; 33(2); 161-73
23. Wakasugi N, Uchida H, Uno S, Safety and effectiveness of once-daily, prolonged-release tacrolimus in de novo kidney transplant recipients: 5-year, multicenter postmarketing surveillance in Japan: Transplant Proc, 2018; 50; 3296-305
24. Pernin V, Glyda M, Viklický O, Long-term prolonged-release tacrolimus-based immunosuppression in de novo kidney transplant recipients: 5-y prospective follow-up of patients in the ADVANCE study: Transplant Direct, 2023; 9(3); e1432
25. Kuypers D, Weekers L, Blogg M, Efficacy of prolonged-release tacrolimus after conversion from immediate-release tacrolimus in kidney transplantation: A retrospective analysis of long-term outcomes from the ADMIRAD study: Transplant Direct, 2023; 9(4); e1465
26. von Elm E, Altman DG, Egger M, The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies: Lancet, 2007; 370; 1453-57
27. Roufosse C, Simmonds N, Clahsen-Van Groningen M, A 2018 reference guide to the Banff classification of renal allograft pathology: Transplantation, 2018; 102; 1795-814
28. Falconer SJ, Peagam WR, Oniscu GC, Early or late conversion from Tac-BD to Tac-BD in renal transplantation: When is the right time?: Transplant Proc, 2015; 47(6); 1741-45
29. Moal V, Grimbert P, Beauvais A, A prospective, observational study of conversion from immediate-to prolonged-release tacrolimus in renal transplant recipients in France: The OPALE study: Ann Transplant, 2019; 24; 517-26
30. Wang TS, Huang KH, Hsueh KC, Efficacy and safety of once-daily prolonged-release tacrolimus versus twice-daily tacrolimus in kidney transplant recipients: A meta-analysis and trial sequential analysis: J Chin Med Assoc, 2023; 86(9); 842-49
31. Auglienė R, Dalinkevičienė E, Kuzminskis V, Factors influencing renal graft survival: 7-year experience of a single center: Medicina (Kaunas), 2017; 53(4); 224-32
32. Pakfetrat M, Malekmakan L, Jafari N, Sayadi M, Survival rate of renal transplant and factors affecting renal transplant failure: Exp Clin Transplant, 2022; 20(3); 265-72
33. Kolonko A, Chudek J, Więcek A, Improved kidney graft function after conversion from twice daily tacrolimus to a once daily prolonged-release formulation: Transplant Proc, 2011; 43; 2950-53
34. Guirado L, Burgos D, Cantarell C, Medium-term renal function in a large cohort of stable kidney transplant recipients converted from twice-daily to once-daily tacrolimus: Transplant Direct, 2015; 1(7); e24
Figures
 Figure 1. Patient flow through the study.
Figure 1. Patient flow through the study. Figure 2. Kaplan-Meier plot of time from transplant to primary composite endpoint event in the full analysis cohort and the 3 tacrolimus conversion cohorts. P-value is based on time to event though 5 years.
Figure 2. Kaplan-Meier plot of time from transplant to primary composite endpoint event in the full analysis cohort and the 3 tacrolimus conversion cohorts. P-value is based on time to event though 5 years. Figure 3. Kaplan-Meier plot of time from transplant to primary composite endpoint event in the full analysis cohort, de novo, and tacrolimus conversion (combined early and late conversion) cohorts. P-value is based on time to event through 5 years.
Figure 3. Kaplan-Meier plot of time from transplant to primary composite endpoint event in the full analysis cohort, de novo, and tacrolimus conversion (combined early and late conversion) cohorts. P-value is based on time to event through 5 years. Figure 4. (A) Mean prescribed tacrolimus dosages from time of transplant, stratified by de novo, early- and late-conversion cohorts. Error bars denote standard error. (B) Mean tacrolimus trough levels from time of transplant, stratified by de novo, early- and late-conversion cohorts. Error bars denote standard error.
Figure 4. (A) Mean prescribed tacrolimus dosages from time of transplant, stratified by de novo, early- and late-conversion cohorts. Error bars denote standard error. (B) Mean tacrolimus trough levels from time of transplant, stratified by de novo, early- and late-conversion cohorts. Error bars denote standard error. Figure 5. Mean (SE) eGFR values from time of transplant, stratified by de novo and combined conversion cohorts (early plus late conversion). Patients with graft loss were included in the analysis of subsequent visits and assigned an eGFR of 0. eGFR – estimated glomerular filtration rate; SE – standard error.
Figure 5. Mean (SE) eGFR values from time of transplant, stratified by de novo and combined conversion cohorts (early plus late conversion). Patients with graft loss were included in the analysis of subsequent visits and assigned an eGFR of 0. eGFR – estimated glomerular filtration rate; SE – standard error. Tables
 Table 1. Baseline characteristics of patients.
Table 1. Baseline characteristics of patients. Table 2. Patient demographics per transplant center.
Table 2. Patient demographics per transplant center. Table 3. Post hoc analysis of outcomes per transplant center.
Table 3. Post hoc analysis of outcomes per transplant center. Table 4. Kaplan-Meier estimates of freedom from the primary composite endpoint stratified by population cohort (first 5 years post-transplant).
Table 4. Kaplan-Meier estimates of freedom from the primary composite endpoint stratified by population cohort (first 5 years post-transplant). Table 5. Sensitivity analysis of primary endpoint: Kaplan-Meier estimates of the individual components of the composite endpoint stratified by population cohort (first 5 years post-transplant).
Table 5. Sensitivity analysis of primary endpoint: Kaplan-Meier estimates of the individual components of the composite endpoint stratified by population cohort (first 5 years post-transplant). Table 6. Kaplan-Meier estimates of patient survival stratified by population cohort (first 5 years post-transplant).
Table 6. Kaplan-Meier estimates of patient survival stratified by population cohort (first 5 years post-transplant). Table 7. Multivariable analysis of independent variables associated with the primary composite endpoint for all patients.
Table 7. Multivariable analysis of independent variables associated with the primary composite endpoint for all patients. Table 8. Laboratory assessments at 8–12 weeks after transplant by population cohort.
Table 8. Laboratory assessments at 8–12 weeks after transplant by population cohort. Table 9. Kaplan-Meier estimates of primary composite endpoint stratified by baseline eGFR.
Table 9. Kaplan-Meier estimates of primary composite endpoint stratified by baseline eGFR. Table 1. Baseline characteristics of patients.
Table 1. Baseline characteristics of patients. Table 2. Patient demographics per transplant center.
Table 2. Patient demographics per transplant center. Table 3. Post hoc analysis of outcomes per transplant center.
Table 3. Post hoc analysis of outcomes per transplant center. Table 4. Kaplan-Meier estimates of freedom from the primary composite endpoint stratified by population cohort (first 5 years post-transplant).
Table 4. Kaplan-Meier estimates of freedom from the primary composite endpoint stratified by population cohort (first 5 years post-transplant). Table 5. Sensitivity analysis of primary endpoint: Kaplan-Meier estimates of the individual components of the composite endpoint stratified by population cohort (first 5 years post-transplant).
Table 5. Sensitivity analysis of primary endpoint: Kaplan-Meier estimates of the individual components of the composite endpoint stratified by population cohort (first 5 years post-transplant). Table 6. Kaplan-Meier estimates of patient survival stratified by population cohort (first 5 years post-transplant).
Table 6. Kaplan-Meier estimates of patient survival stratified by population cohort (first 5 years post-transplant). Table 7. Multivariable analysis of independent variables associated with the primary composite endpoint for all patients.
Table 7. Multivariable analysis of independent variables associated with the primary composite endpoint for all patients. Table 8. Laboratory assessments at 8–12 weeks after transplant by population cohort.
Table 8. Laboratory assessments at 8–12 weeks after transplant by population cohort. Table 9. Kaplan-Meier estimates of primary composite endpoint stratified by baseline eGFR.
Table 9. Kaplan-Meier estimates of primary composite endpoint stratified by baseline eGFR. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








