20 February 2024: Original Paper
Lymphocele Outcomes After Renal Transplantations Performed by an Experienced Surgeon: Is Meticulously Performed Surgery and Experience Adequate to Prevent Lymphocele?
Nurettin Ay1ABCDEF*, Vahhac Alp1B, Recai Duymuş2BD, Sedat Çetin3BDDOI: 10.12659/AOT.942656
Ann Transplant 2024; 29:e942656
Abstract
BACKGROUND: The purpose of the present study was to analyze the rate of lymphoceles in kidney transplant operations meticulously performed by the same senior surgeon.
MATERIAL AND METHODS: The present study included 315 patients who were operated on in our organ transplantation center and followed up in the polyclinic after July 2013. The patients were retrospectively divided into 2 groups: patients with and without lymphocele. Symptomatic lymphocele (SL) has been defined as symptomatic fluid collection around the graft that necessitates an intervention for the graft or patient.
RESULTS: Lymphocele was observed in 82 (26%) patients. An intervention was needed in 16 (5.1%) of these cases. Demographic data such as age and sex of both groups were similar. Lymphocele cases were mostly asymptomatic, with a size <6 cm (75.6%). However, intervention was needed in 16 (75%) of the patients with a size ≥6 cm that were symptomatic. The length of time on dialysis in the pretansplant period was shorter in the group that developed lymphocele, and a lower rate of graft loss was observed in these patients. No statistically significant difference was found between the 2 groups in terms of rejection rates, serum albumin/globulin levels, and development of de novo DSA.
CONCLUSIONS: The risk factors reported in the literature related with lymphocele formation were not found to be statistically significant in our study. Complications, except lymphocele, were observed less frequently, but lymphocele formation was encountered in our patients despite meticulous surgery.
Keywords: Lymphocele, Kidney Transplantation, Risk Factors
Background
The most common surgical complications observed after kidney transplantation are lymphocele, urinoma, and hematomas. Lymphocele is a pseudocystic formation filled with lymphatic fluid around the graft in kidney transplant recipients. Its mean incidence rate is 5.2%, but varies from 0.03% to 51%). The diagnosis of lymphocele is usually made by ultrasonography (USG) between the postoperative 2nd week and 6th month. The highest incidence is encountered at the 6th week. However, there is also a case which was reported at 3.7 years after surgery [1–10].
The most important risk factor for postransplant lymphocele (PTL) is the lymphatic injury that occurs during vascular isolation of the iliac vessels. The other known risk factors are advanced age, obesity, urinary tract malformation, autosomal dominant polycystic kidney disease (ADPKD), diabetes mellitus, delayed graft function (DGF), mammalian target of rapamycin (mTOR) inhibitors, diuretic drugs, steroids, acute rejection, renal capsular injury, and donor type [8,10,11].
PTL cases that are asymptomatic are usually diagnosed during routine USG follow-ups. However, symptomatic patients may have pain and tenderness in the right lower quadrant due to compression effect, compression on the urinary bladder and ureter, deep vein thrombosis, ipsilateral extremity edema, infection, and graft loss. In treatment, aspiration and drainage are performed under USG guidance, while laparoscopic or open fenestration is applied in resistant cases [12–14].
Kidney transplantation is a surgical procedure that should be performed by an experienced surgical team. The differences between complications resulting from surgical technique can be minimized by having the same surgeon perform the kidney transplant operations (the primary surgeon who performs the transplantation procedure). The purpose of the present study was to analyze the rate of lymphocele in the kidney transplant operations meticulously performed by the same senior surgeon.
Material and Methods
STUDY DESIGN AND STUDY POPULATION:
The present study included 315 patients who were operated on in our organ transplantation center after July 2013 and followed up in the polyclinic. The approval of local ethics committee was obtained to report the outcomes of our recipients (Gazi Yaşargil Training and Research Hospital, Ethics Commission, Dated 14.07.2023 and numbered 457). USG was performed by a specialist radiologist at least once before discharge in the early postoperative period. Additionally, Doppler USG was performed daily by the transplantation surgeon. After patient discharge, abdominal USG and Doppler USG were performed once weekly in the first month, once biweekly between the 2nd and 3rd months, once monthly between the 4th and 6th months, and thereafter at least once a year. Patients were retrospectively divided into 2 groups as those with and without lymphocele. Patients who developed lymphocele were classified as those with a size <3 cm, 3–5.9 cm, 6–9.9 cm, and >10 cm. Symptomatic lymphocele (SL) was defined as symptomatic fluid collection around the graft that necessitated an intervention for the graft or patient.
A drainage catheter was applied in patients with symptomatic lymphocele or lymphocele >10 cm by interventional radiology under USG guidance. Patients were re-evaluated by USG after drainage was completed. We removed the drainage catheter if no fluid collection was present. However, the drainage catheter was closed in patients in whom lymphocele continued longer than 2 weeks or when recurrent drainage procedures were needed, and these patients were monitored by USG for 3 days. Laparoscopic fenestration was performed in the cases detected with fluid collection. The cases not detected with fluid collection were monitored with a closed drain for 1 week and the drain was withdrawn if no fluid collection was found by control USG.
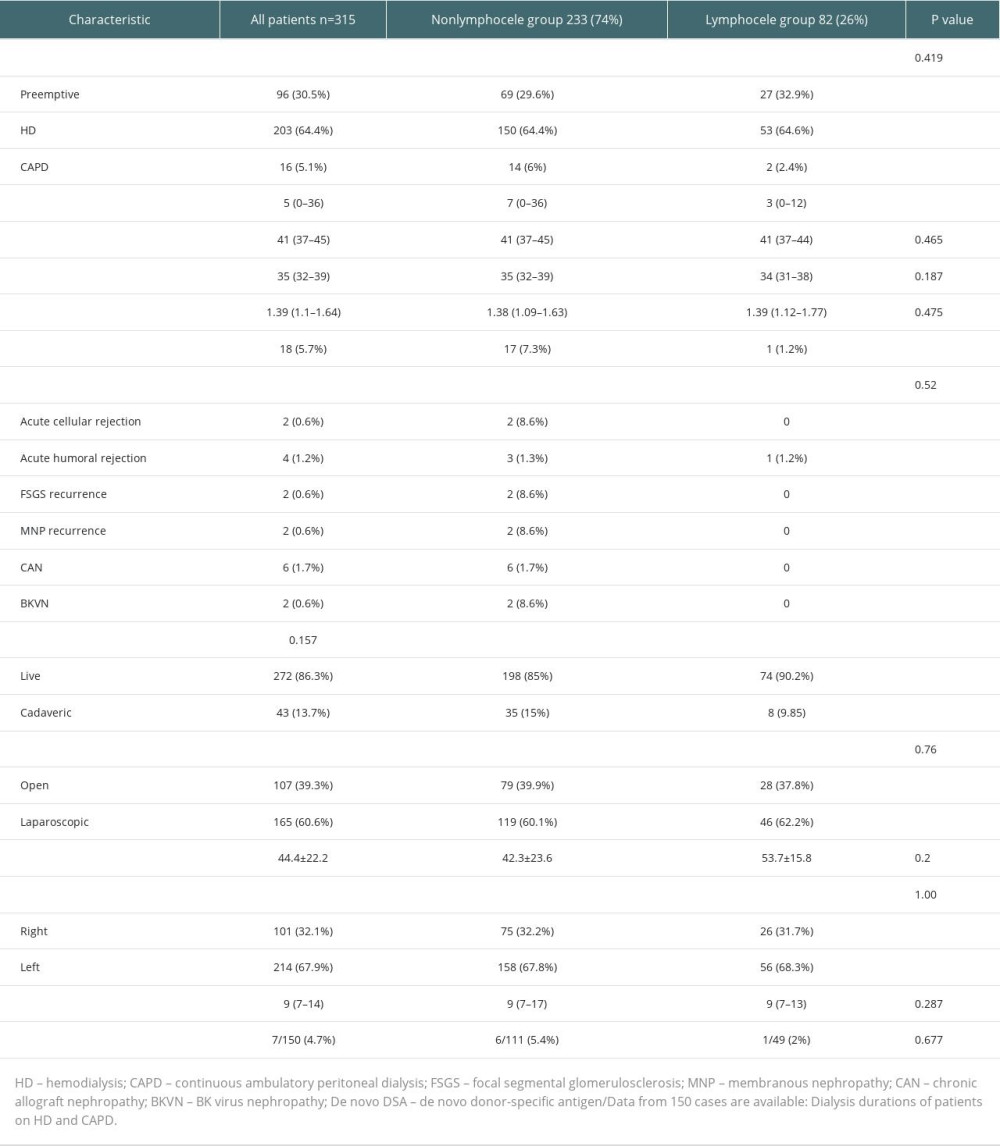
The patients who did and did not develope PTL were compared in terms of age at transplantation, sex, the cause of end-stage renal disease (ESRD), the intervention performed for lymphocele, the technique applied for vascular isolation, body mass index (BMI), administered induction and maintenance immunosuppressive treatments, mismatch (MM) count, whether rejection developed, preoperative dialysis method, length of time on dialysis, albumin levels assessed preoperatively and at discharge, albumin/globulin ratio at discharge, donor type, donor operation procedure, side of the transplanted kidney, donor age, length of hospital stay, and levels of de novo donor-specific antigen (de novo DSA; data of only 150 patients were available).
KIDNEY TRANSPLANTATION:
All donor operations were performed by 2 experienced senior surgeons. Laparoscopic and open donor nephrectomy were applied in 165 and 107 cases, respectively. There were 43 cadaveric kidney transplantations. All recipient operations were performed by a senior surgeon who was adequately trained for kidney transplantation. The senior surgeon had 20 years of transplantation experience and performed the transplant procedure during the period between the dates indicated in this study. The retroperitoneal space was entered via a Gibson incision. The abdominal organs were medialized over the psoas muscle without opening the peritoneum. External/common iliac arteries and lymphatic tissue around the vein were dissected and made appropriate for anastomosis. Two separate techniques were applied for this procedure – lymphatic veins crossing the external iliac artery or vein were ligated using 3/0 polyglactin at both ends and cut by Metzenbaum or monopolar electrocautery in the first technique; and lymphatic veins were detached from the vascular structures using the right-angle clamp and dissected after coagulating at a sufficient width using penset and monopolar cautery in the second technique. After completion of donor nephrectomy, cold perfusion was applied using histidine-tryptophan-ketoglutarate (HTK) and University of Wisconsin solutions for alive and cadaver kidney donors in the back-table, respectively. In the back-table operation, all lymphatic tissues around the arterial and venous vessels were meticulously dissected and ligated by the senior surgeon who performed the recipient operation. The graft kidney was taken to the recipient operating table and ureteroneocystostomy was performed by making vascular anastomoses using 6/0 polypropylene and the anastomosis between the ureter and urinary bladder using 6/0 polydioxanone suture with Lich-Gregoir technique. Double-J stents were used for ureteroneocystostomy anastomosis. None of the cases required laparotomy. Renal transplantation was performed in the iliac fossa of the contralateral region because of iliac thrombus in 3 cases. A drain was inserted into the posterior of the kidney graft in all cases. In the postoperative period, the drainage catheter was removed when drain output was below 50 mL for 2 consecutive days. In patients without symptomatic lymphocele, the double-j stent was withdrawn on the 21st postoperative day.
IMMUNOSUPPRESSIVE THERAPY:
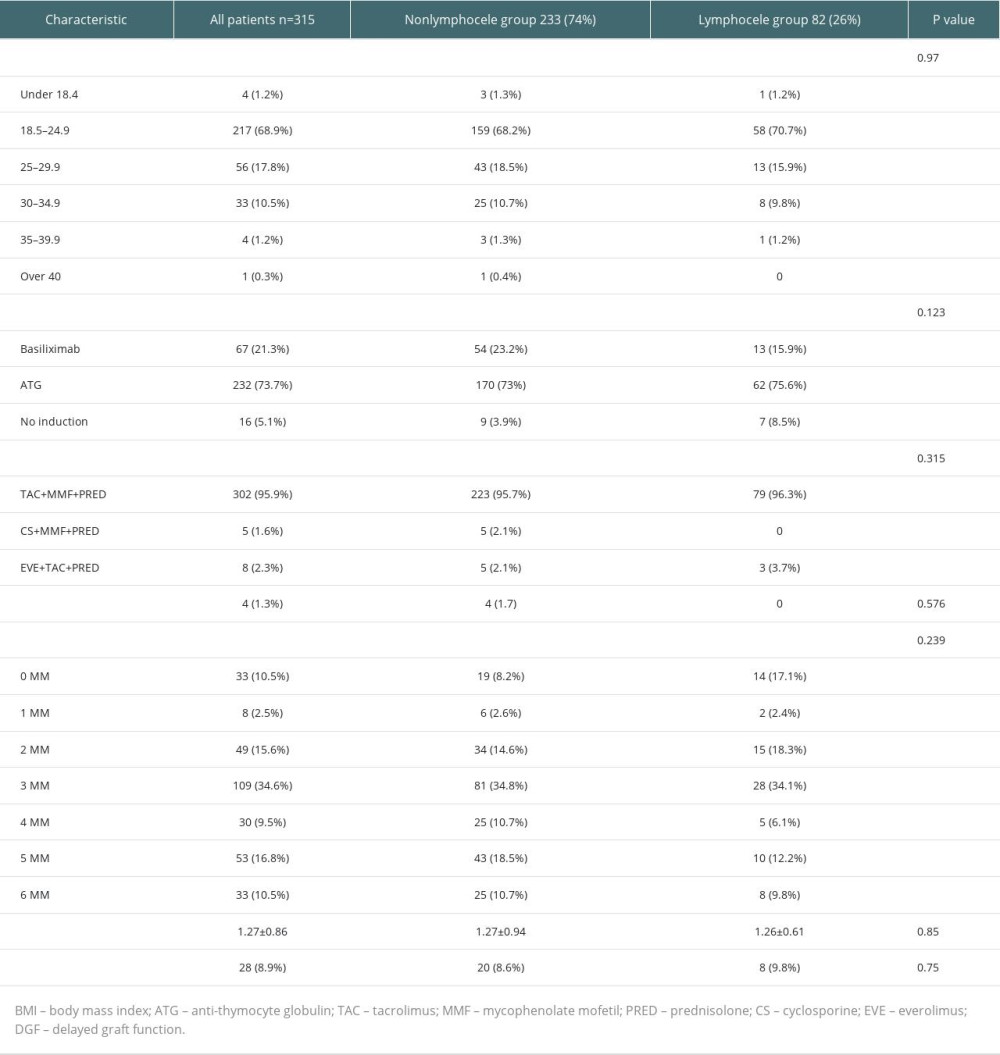
A triple immunosuppressive protocol (tacrolimus, mycophenolic acid, and methylprednisolone) was administered in all cases. Anti-thymocyte globulin (ATG) was given as the induction agent at a dose of 2 mg/kg for 3 days in all cases who had a risk factor. No induction agent was used in the cases with mismatch count of 0. In the remaining cases, 20 mg basiliximab was administered as the induction agent on day 0 and day 4. Therapy protocol was changed to everolimus or cyclosporine in patients who developed intolerance to tacrolimus or mycophenolic acid. Methylprednisolone 1000 mg was administered intraoperatively. Methylprednisolone dose was reduced by half every day and 20 mg oral prednisolone was started on the 6th postoperative day for daily use. The oral prednisolone dosage was gradually reduced to 5 mg a day within the first post-transplant year. Calcineurin inhibitors (tacrolimus or cyclosporine) and mycophenolate sodium (MMF; 1440 mg a day, as 2 divided doses) were used as maintenance immunosuppressants. MMF was used at a dose of 600 mg/m2 as 2 divided doses in children.
STATISTICAL ANALYSIS:
All statistical analyses were performed using the IBM SPSS software (IBM SPSS Statistics for Windows, Version 24.0, Armonk, NY: IBM Corp.). Continuous variables are presented as median and interquartile range and categorical variables are presented as counts and percentages. The Kolmogorov-Smirnov test was used to evaluate the distribution normality of the continuous variables. Continuous variables were compared with the
Results
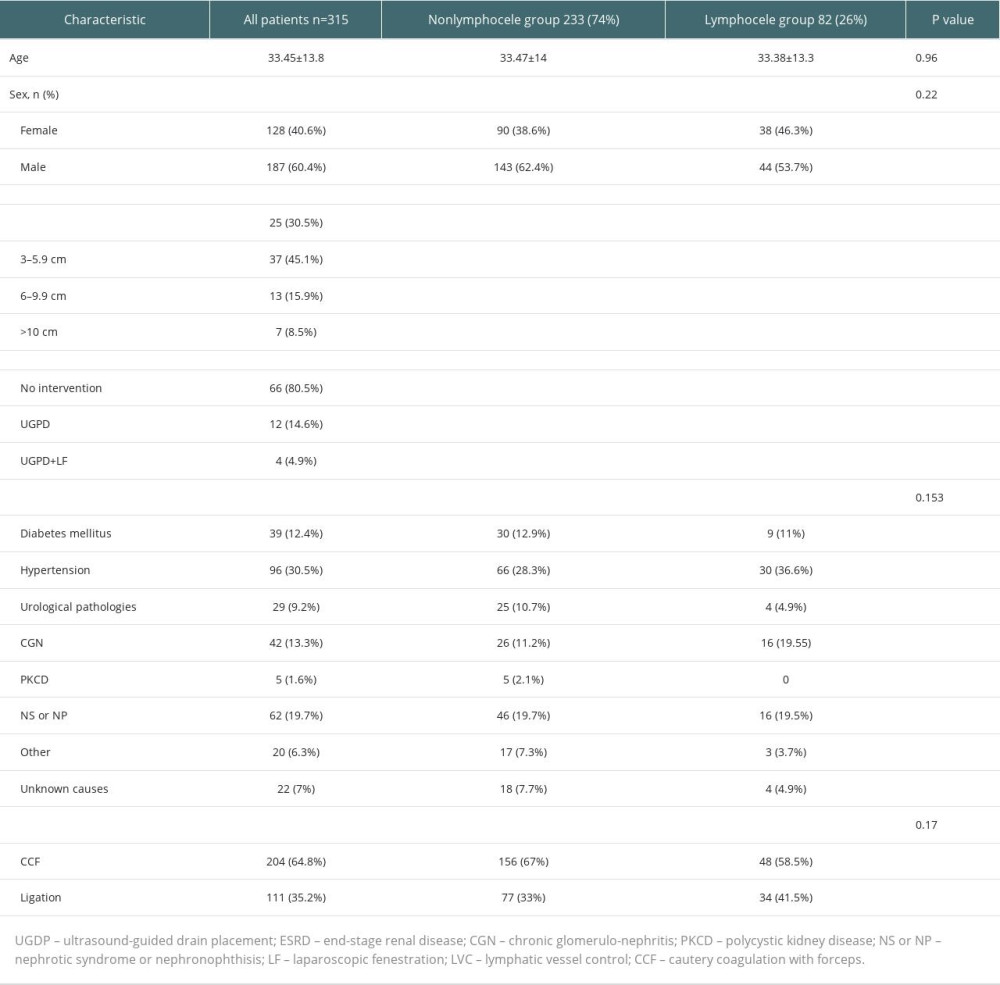
The study included 315 patients who underwent renal transplantation in our center between August 2013 to January 2023 and were followed up in the polyclinic. Lymphocele was discovered in 82 (26%) patients. An intervention was needed in 16 (5.1%) of these patients. SL was diagnosed at a mean 26.3±16 postoperative days (range, 6–52 days). Only 1 patient was admitted at the 5th postoperative year because of SL causing compression on the ureter and high Cr levels within the first month after an motor vehicle injury that was not encountered in the US images before. Demographic data such as age and sex of both groups were similar (Table 1). Lymphocele cases were mostly asymptomatic, with a size <6 cm (75.6%). However, intervention was needed since 16 (75%) of the patients with a size ≥6 cm who were symptomatic. The reasons for interventions were compression on the ureter and/or high creatine level in all cases. Hypertension was the most common cause of ESRD. No statistically significant difference was found between the groups in terms of all known etiologies, including diabetes mellitus (
We detected no impact of receiving dialysis therapy in the preoperative period or dialysis type on lymphocele formation (Table 3). However, the length of time on dialysis in the pre-transplant period was significantly shorter in the lymphocele group (
The patients were also evaluated for other complications requiring postoperative surgery. The most common complication was postoperative incisional hernia in 3 cases (0.9%). Subcapsular hematoma, bleeding, renal artery pseudoaneurysm, and ureteroneocystostomy obstruction were observed in 1 patient each.
Discussion
Lymphoceles are the pseudocysts filled with lymphatic fluids that form around the kidneys after kidney transplantation [16]. In the literature, very different rates have been reported related with its incidence resulting from the different definitions. Lymphocele was observed in 82 (26%) patients of our cohort. An intervention was performed in only 16 (5.07%) of those. In the literature, the incidence of SL was reported to be 5.2% [17]. This rate was consistent with our cohort. Although the etiology of lymphocele is unclear, it is considered result from impaired lymphatic flow in the graft or donor. Risk factors other than surgical dissection include use of high-dose corticosteroids, immunosuppressive drugs, cellular rejection, obesity, diabetes mellitus, and PCKD [18–20]. In another recent study, age (50–65 years), PCKD, and need for a drainage catheter were determined to be independent risk factors for kidney transplantations performed by an experienced surgeon or under supervision of this surgeon [21]. In our study, we detected an increased rate of lymphocele due to none of the risk factors mentioned above. However, we could not evaluate the effect of a drainage catheter on the formation of lymphocele since we routinely use drainage catheters in all our patients.
There are studies in the literature that have asserted acute rejection increases the formation of lymphocele through a mechanism similar to inflammation by activating lymphatic angiogenesis [22,23]. Lehner et al found that development of rejection (cellular+humoral) and delayed graft function (DGF) were related with lymphocele formation, but they have detected no relationship between development of de novo DSA and lymphocele formation [24]. We discovered no relationship of DGF and acute rejection with lymphocele formation in our study.
We attempted to develop standardizations to decrease the surgical complications during our experience of transplantation. Therefore, all stages of the transplantation, including meticulous ligation of the lymphatic tissue in the graft after donor nephrectomy and lymphatic dissection of the recipient operation, were performed by the same surgeon. During vascular isolation, the lymphatics were ligated via meticulous coagulation using penset and cautery or polyglactin suture. Dubeaux et al found that meticulous ligation of lymphatics at the back-table stage of donor kidney and implantation significantly (0.6%) reduces the lymphocele rate. Another study found that laparoscopic donor nephrectomy can decrease the frequency of lymphocele. In addition, it has been reported that use of bipolar cautery decreased the formation of lymphocele [25–27]. Serirodoma compared ligation and use of only monopolar cautery for lymphatic dissection and found that lymphocele rates were significantly lower in the ligation group (5.2% vs 11.8,
Sayilar et al [29] found a lower serum albumin level in the pretansplant period. However, there was no significant difference between serum albumin levels at time of diagnosis and pre-transplant period in the patients diagnosed with lymphocele. They explained the decreased concentration of serum albumin in the post-transplant period with loss of protein-rich lymphatic fluid. In the same study, preemptive kidney transplantation and dialysis type were not effective in lymphocele formation. Although we found a clinically lower level of serum albumin at time of diagnosis of lymphocele, we found no relationship between pre-transplant and post-transplant levels of serum albumin and formation of lymphocele. We also found no impact of serum albumin/globulin ratio that may be an indicator of inflammation on the formation of lymphocele. No significant difference was present between the types of dialysis in both groups. However, detection of a lower rate of graft loss and shorter length of time on dialysis (it was weakly statistically significant) in the lymphocele group was an unexplainable outcome. Pacovsky found that a serum albumin level that is potentially critical regarding the pathogenesis of post-transplant lymphocele was 44.1 g/L (however, the sensitivity level was only 62%), and they suggested that the essential risk factor for formation of lymphocele was still the skill of the surgeon [30]. We did not observe a significant difference in lymphocele formation when we changed our vascular isolation technique. We also observed that none of the techniques we applied prevented lymphocele formation. This may be due to the lymphatics that we could not control in the retroperitoneal space.
Our study has some limitations. First, there may be unmeasured factors that influenced the association between kidney transplantation and lymphocele formation. Second, our study’s retrospective design, single-center nature, relatively small sample size, and missing de novo DSA data may limit the scope of our results.
Conclusions
Striving to achieve perfection in surgical technique and reducing postoperative complications is the surgeon’s goal. The risk factors reported in the literature related with lymphocele formation were not found to be statistically significant in our study. However, complications, except lymphocele, were observed less frequently, and lymphocele formation were encountered in our cases despite meticulous surgery.
References
1. Ranghino A, Segoloni GP, Lasaponara F, Biancone L, Lymphatic disorders after renal transplantation: New insights for an old complication: Clin Kidney J, 2015; 8(5); 615-22
2. Minetti EE, Lymphocele after renal transplantation, a medical complication: J Nephrol, 2011; 24; 707-16
3. Di Carlo HN, Darras FS, Urologic considerations and com plications in kidney transplant recipients: Adv Chronic Kidney Dis, 2015; 22; 306e11
4. Bzoma B, Kostro J, Debska-Slizien A, Treatment of the lymphocele after kidney transplantation: A single-center experience: Transplant Proc, 2016; 48; 1637-40
5. Zietek Z, Sulikowski T, Tejchman K, Lymphocele after kidney transplantation: Transplant Proc, 2007; 39; 2744-47
6. De Lima ML, Cotrim CAC, Moro JC, Laparoscopic treatment of lymphoceles after renal transplantation: Int Braz J Urol, 2012; 38; 215-21
7. Ulrich F, Niedzwiecki S, Fikatas P, Symptomatic lymphoceles after kidney transplantation – multivariate analysis of risk factors and outcome after laparoscopic fenestration: Clin Transplant, 2010; 24; 273-80
8. Golriz M, Klauss M, Zeier M, Mehrabi A, Prevention and management of lymphocele formation following kidney transplantation: Transplant Rev (Orlando), 2017; 31(2); 100e5
9. Lucewicz A, Wong G, Lam VW, Management of primary symptomatic lymphocele after kidney transplantation: A systematic review: Transplantation, 2011; 92; 663-73
10. Heer MK, Clark D, Trevillian PR, Functional significance and risk factors for lymphocele formation after renal transplantation: ANZ J Surg, 2018; 88(6); 597-602
11. Sim A, Ng LG, Cheng C, Occurrence of a lymphocele following renal transplantation: Singapore Med J, 2013; 54(5); 259-62
12. Bailey SH, Mone MC, Holman JM, Nelson EW, Laparoscopic treatment of post renal transplant lymphoceles: Surg Endosc, 2003; 17(12); 1896-99
13. Zagdoun E, Ficheux M, Lobbedez T, Complicated lymphoceles after kidney transplantation: Transpl Proc, 2010; 42; 4322-25
14. Fuller TF, Kang SM, Hirose R, Management of lymphoceles after renal transplantation: Laparoscopic versus open drainage: J Urol, 2003; 169(6); 2022-25
15. Ay N, Alp V, Kaya Ş, Posttransplant de novo donor specific HLA antibody monitoring and clinical outcomes: A single-center experience: The European Research Journal, 2021; 7(3); 304-11
16. Ebadzadeh MR, Tavakkoli M, Lymphocele after kidney transplantation: Where are we standing now?: Urol J, 2008; 5; 144-48
17. Joosten M, d’Ancona FC, van der Meijden WA, Poyck PP, Predictors of symptomatic lymphocele after kidney transplantation: Int Urol Nephrol, 2019; 51; 2161-67
18. Nelson EW, Gross ME, Mone MC, Does ultrasonic energy for surgical dissection reduce the incidence of renal transplant lymphocele?: Transplant Proc, 2011; 43; 3755-59
19. Hamza A, Fischer K, Koch E, Diagnostics and therapy of lymphoceles after kidney transplantation: Transplant Proc, 2006; 38; 701-6
20. Mehrabi A, Fonouni H, Kashfi A, The role and value of sirolimus administration in kidney and liver transplantation: Clin Transplant, 2006; 20(Suppl 17); 30-43
21. Sevmis M, Aktas S, Alkarab U, Risk factors, diagnosis, and treatment of lymphocele after renal transplantation: A retrospective study: Transplant Proc, 2021; 53; 1040-47
22. Stuht S, Gwinner W, Franz I, Lymphatic neoangiogenesis in human renal allografts: Results from sequential protocol biopsies: Am J Transplant, 2007; 7; 377-84
23. Kerjaschki D, Regele HM, Moosberger I, Lymphatic neoangiogenesis in human kidney transplants ıs associated with ımmunologically active lymphocytic ınfiltrates: J Am Soc Nephrol, 2004; 15; 603-12
24. Lehner LJ, Hohberger A, Marschke L, Analysis of risk factors and long-term outcomes in kidney transplant patients with ıdentified lymphoceles: J Clin Med, 2020; 9; 2841
25. Dubeaux VT, Oliveira RM, Moura VJ, Assessment of lymphocele incidence following 450 renal transplantations: Int BrazJ Urol, 2004; 30(1); 18-21
26. Simforoosh N, Basiri A, Tabibi A, Comparison of laparoscopic and open donor nephrectomy: A randomized controlled trial: BJU Int, 2005; 95(6); 851-55
27. Simforoosh N, Tabibi A, Rad HM, Gholamrezaie HR, Comparison between bipolar lymphatic vessels cautery and suture ligature in prevention of postrenal transplant lymphocele formation: A randomized controlled trial: Exp Clin Transplant, 2019; 17(1); 26-30
28. Serirodoma M, Taweemonkongsapa T, Chotikawanicha E, Lymphocele in kidney transplantation: A comparison of ligation and non-ligation technique of iliac lymphatic dissection: Transplantat Proc, 2022; 54; 2197-204
29. Sayilar EI, Ersoy A, Ayar Y, Factors influencing lymphocele development after kidney transplant: Single center experience: Exp Clin Transplant, 2022; 21; 116-22
30. Pacovskya J, Hysplerc R, Huseka P, Colloid osmotic pressure participates on the post-transplant lymphocele pathogenesis: Transplant Proc, 2018; 50; 3422-25
In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860