05 March 2024: Original Paper
No Prognostic Impact of Graft-to-Recipient Weight Ratio on Hepatocellular Carcinoma Recurrence Following Living Donor Liver Transplantation
Geunhyeok YangDOI: 10.12659/AOT.942767
Ann Transplant 2024; 29:e942767
Abstract
BACKGROUND: The effects of a low graft-to-recipient weight ratio (GRWR) on the prognosis of patients with hepatocellular carcinoma (HCC) are unclear. The present study examined whether the GRWR had an impact on the rate of HCC recurrence following living donor liver transplantation (LDLT).
MATERIAL AND METHODS: This retrospective observational single-center study included 856 patients who underwent LDLT for HCC between January 2006 and December 2016 at Asan Medical Center and evaluated the association between GRWR and post-transplant tumor recurrence.
RESULTS: Of the 856 patients who underwent LDLT for HCC, 54 (6.3%), 272 (31.8%), 274 (32.0%), and 256 (29.9%) had GRWR <0.8%, 0.8-0.99%, 1.0-1.19%, and ≥1.2%, respectively. Analysis of all patients revealed that the disease-free survival (DFS; P=0.545) and overall survival (OS; P=0.313) rates were not different in these 4 groups. Subgroups analyses also showed that GRWR did not influence survival rates in patients within (DFS: P=0.398; OS: P=0.676) and beyond (DFS: P=0.602; OS: P=0.649) the Milan criteria, or in patients with α-fetoprotein–des-γ-carboxyprothrombin–tumor volume scores <5log (DFS: P=0.633; OS: p=0.285) and ≥5log (DFS: P=0.674; OS: P=0.906).
CONCLUSIONS: GRWR less than 0.8% did not demonstrate a noteworthy prognostic influence on the oncological results among patients who had undergone LDLT for HCC. High-volume multi-center studies are necessary to validate these findings.
Keywords: Liver Neoplasms, Liver Transplantation, Living Donors, Neoplasm Recurrence, Local, Patient Selection
Background
Hepatocellular carcinoma (HCC) is a well-established indication for liver transplantation (LT) and is a significant reason for living donor liver transplantation (LDLT) in Asian countries [1]. Nevertheless, HCC recurrence after LT leads to reduced patient survival, even with aggressive approaches to recurrent tumor management. Consequently, the selection of candidates for LT is conducted with meticulous consideration to minimize the likelihood of HCC recurrence.
Post-transplant HCC recurrence rates have been reported to be higher in patients undergoing LDLT than in deceased donor LT [2,3]. The higher recurrence rates in patients undergoing LDLT may be due to the expedited process of patient evaluation and selection for LDLT, resulting in transplantation of more aggressive tumors [3]; excessive manipulation of the native liver for preservation of the retrohepatic inferior vena cava [4]; and regeneration of the partial liver graft, enhancing HCC recurrence [5].
A single-center study from Korea reported that a graft-to-recipient weight ratio (GRWR) <0.8% was associated with higher tumor recurrence rates in patients with HCC beyond, but not within, the Milan criteria [5]. By contrast, other studies found that small-sized liver grafts do not raise the risk of HCC recurrence after LDLT [6,7]. Several animal studies, however, have indicated that regeneration of the partial liver graft enhances HCC recurrence [8–10].
To address the conflicting findings concerning the prognostic impact of GRWR, this retrospective, single-center, observational study aimed to investigate whether GRWR played a role in influencing the occurrence of HCC recurrence following LDLT.
Material and Methods
PATIENT SELECTION:
This retrospective observational single-center study evaluated the association between GRWR and post-transplant HCC recurrence. We conducted a search within the LT database of the Asan Medical Center to find adult patients who had undergone primary LDLT for HCC between January 2006 and December 2016, covering 11 years. Inclusion criteria were patients aged ≥18 years at the time of LDLT, with a pre-LT diagnosis of HCC. Exclusion criteria were patients incidentally diagnosed with HCC through liver examination at the time of transplantation, those who had undergone re-transplantation, individuals with combined HCC-cholangiocarcinoma, and those who had died within less than 3 months after LT. Additionally, patients who had undergone dual-donor LT were excluded to prevent potential bias associated with multiple donors. A total of 856 patients were included in this study.
PATIENT GROUPING AND FOLLOW-UP:
Small-sized liver grafts usually indicate those with GRWR <0.8%, but the range of GRWR ≥0.8% in the control group was too wide to compare objectively. Thus, these patients were divided into 4 GRWR groups, consisting of patients with GRWR <0.8%, ≥0.8% and <1.0%, ≥1.0%, and <1.2%, and ≥1.2% [6]. Patients were also divided into 2 groups based on the Milan criteria, consisting of those within and beyond the Milan criteria; and into those based on α-fetoprotein–des-γ-carboxy prothrombin–tumor volume (ADV) scores, with a cutoff of 5log [11–13]. The mammalian target of rapamycin inhibitors were not administered before HCC recurrence in the study patients. Patient follow-up was extended until April 2021 or until the time of patient death, and this was accomplished by comprehensive review of institutional medical records, along with support from the National Health Insurance Service in Korea.
ETHICS STATEMENT:
The study protocol received approval from the institutional review board of Asan Medical Center (No. 2021-1611), which waived the need for informed consent given the retrospective nature of this investigation. The study adhered to ethics guidelines outlined in the World Medical Association Declaration of Helsinki 2013.
STATISTICAL ANALYSIS:
Numerical data were expressed as either median with corresponding ranges or as mean with standard deviations (SD). Continuous variables underwent comparisons via the
Results
PATIENT DEMOGRAPHICS:
The 856 patients included in the present study were classified into 4 groups according to the GRWR values, with 54 (6.3%), 272 (31.8%), 274 (32.0%), and 256 (29.9%) patients having GRWRs <0.8%, 0.8–0.99%, 1.0–1.19%, and ≥1.2%, respectively. The clinicopathologic features of these 4 groups were similar, except for mean patient age and the proportions of patients who underwent ABO-incompatible LT (Table 1). Because these 2 factors are not considered significant risk factors for HCC recurrence, propensity score matching was not performed.
DISEASE-FREE AND OVERALL PATIENT SURVIVAL:
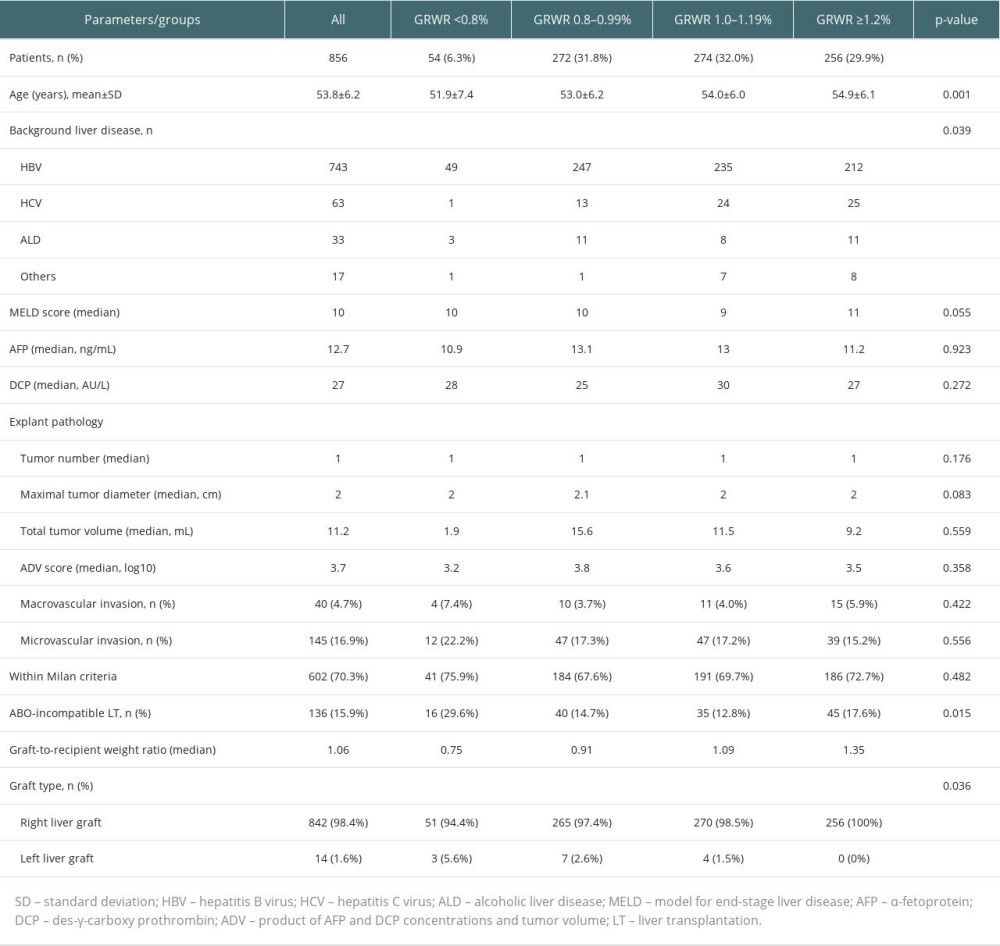
Out of the 856 patients included in the study, HCC recurrence was observed in 179 patients (20.9%), while 123 patients (14.4%) died due to various causes during a median follow-up duration of 79 months. Among the 123 patients who died, 98 died due to HCC recurrence-related reasons, while the remaining 25 died due to other causes. The 1-, 3-, 5-, and 10-year disease-free survival (DFS) rates were 88.0%, 81.7%, 79.1%, and 77.7%, respectively, whereas the 1-, 3-, 5-, and 10-year overall survival (OS) rates were 96.6%, 88.7%, 86.7%, and 84.2%, respectively (Figure 1).
DISEASE-FREE AND OVERALL PATIENT SURVIVAL ACCORDING TO GRWR:
Evaluation of the effects of GRWR on patient survival outcomes revealed that the 5-year DFS rates in patients with GRWRs <0.8%, 0.8–0.99%, 1.0–1.19%, and ≥1.2% did not differ significantly, being 83.5%, 76.7%, 79.6%, and 80.2%, respectively (P=0.545) (Figure 2). Similarly, there were no significant differences observed in the 5-year OS rates among these 4 groups, being 86.9%, 82.9%, 89.7%, and 87.2%, respectively (P=0.313) (Figure 2).
DISEASE-FREE AND OVERALL PATIENT SURVIVAL ACCORDING TO GRWR IN PATIENTS WITHIN AND BEYOND MILAN CRITERIA:
Of the 856 patients included in this study, 602 were within the Milan criteria and 254 were beyond the Milan criteria. Assessment of the 602 patients with HCC within the Milan criteria showed that the 5-year DFS rates of those with GRWRs <0.8%, 0.8–0.99%, 1.0–1.19%, and ≥1.2% were 92.0%, 86.8%, 85.6%, and 88.9%, respectively (P=0.398), and that their 5-year OS rates were 92.6%; 89.1%, 92.6%, and 91.0%, respectively (P=0.676) (Figure 3).
Assessment of the 254 patients with HCC beyond the Milan criteria showed that the 5-year DFS rates of those with GRWRs <0.8%, 0.8–0.99%, 1.0–1.19%, and ≥1.2% were 51.3%, 55.7%, 65.5%, and 56.9%, respectively, whereas their 5-year OS rates were 67.7%, 70.0%, 83.1%, and 77.0%, respectively (P=0.649) (Figure 4).
DISEASE-FREE AND OVERALL PATIENT SURVIVAL ACCORDING TO GRWR WITH ADV SCORE CUTOFF AT 5LOG:
Of the 856 patients included in this study, 661 had ADV scores <5log and 195 had ADV scores ≥5log. Assessment of the 661 patients with ADV scores <5log revealed that the 5-year DFS rates of those with GRWRs <0.8%, 0.8–0.99%, 1.0–1.19%, and ≥1.2% were 92.1%, 85.8%, 87.9%, and 86.6%, respectively (P=0.633); whereas their 5-year OS rates were 94.8%, 87.9%, 95.2%, and 91.6%, respectively (P=0.285) (Figure 5).
Assessment of the 195 patients with ADV scores ≥5log revealed that the 5-year DFS of those with GRWRs <0.8%, 0.8–0.99%, 1.0–1.19%, and ≥1.2% were 65.2%, 50.8%, 50.7%, and 51.2%, respectively (P=0.674), and that their 5-year OS rates were 71.1%, 68.6%, 70.9%, and 67.7%, respectively (P=0.906) (Figure 6).
Discussion
Forecasting the post-transplant prognosis in individuals who have received LT as a treatment for HCC poses a challenge, given the considerable variation in tumor burden, the heterogeneous nature of HCC tumor biology, and the potential impact of immunosuppressive therapy on outcomes. Previous studies have explored various factors to predict the likelihood of HCC recurrence after LT, including tumor size, number of tumors, vascular invasion, tumor differentiation, and levels of alpha-fetoprotein and des-γ-carboxy prothrombin [14–17]. Careful patient selection for LT has been suggested to mitigate the risk of post-transplant HCC recurrence [17–19].
Post-transplant HCC recurrence rates have been reported to be higher in patients who undergo LDLT than those who receive deceased donor LT [2,3], and one of the underlying causes of this difference was thought to be regeneration of the partial liver graft in LDLT, which enhances HCC recurrence [5]. A Korean single-center study that included 428 patients who underwent LDLT showed that GRWR <0.8% was associated with higher tumor recurrence rates in patients with HCC beyond, but not within, the Milan criteria [5]. Several animal studies have suggested that regeneration of the partial liver graft encourages HCC recurrence [8–10]. For instance, prior studies have indicated substantial activation of cellular signaling pathways associated with tumor invasion and migration in recipients of small-for-size liver grafts, potentially promoting increased tumor growth and metastasis following LT [8].
Other studies have reported that small-sized liver grafts do not raise the risk of HCC recurrence after LDLT [6,7]. A previous study from our center analyzed the influence of GRWR on HCC recurrence in 181 LDLT recipients with HCC within the University of California at San Francisco criteria. These patients were divided into 4 GRWR groups, consisting of those with low (<0.8%), middle (0.8–1.0%), and high (>1.0%) GRWR, and those who received whole liver grafts (GRWR >1.5%). A GRWR less than 0.8% was not a substantial risk factor for HCC recurrence, suggesting that a smaller living donor liver graft and the subsequent liver regeneration did not elevate the risk of HCC recurrence following LT, particularly when HCC satisfied the Milan or UCSF criteria [6]. An analysis of 295 patients in the China Liver Transplant Registry reported that small grafts, with GRWR ≤0.8%, can predict inferior survival outcomes but cannot predict earlier HCC recurrence [7].
The present study found that GRWR <0.8% was not prognostic for HCC recurrence or patient survival. Subgroup analyses also showed that GRWR <0.8% was not a significant risk factor for survival outcomes in patients within and beyond the Milan criteria, similar to findings in our previous study [6]. Subgroup analyses were also performed using an ADV score cutoff of 5log, which was shown to be more prognostic than the Milan criteria [12]. GRWR <0.8% was not found to be a significant risk factor for DFS or OS in patients with ADV scores <5log and ≥5log. Taken together, these findings indicate that GRWR <0.8% is not prognostic for post-transplant HCC recurrence. However, the proportion of patients with GRWR <0.8% was only 6.3% in the present study; thus, special attention should be paid to the interpretation of the results.
Although GRWR >0.8% is preferable, LDLT with GRWR between >0.6% and 0.8% has been performed if an alternative living donor is unavailable. This type of small-for-size graft has been associated with the development of small-for-size syndrome. Portal vein pressure was significantly higher in patients receiving small-sized liver grafts [20,21]. Although elevated portal venous pressure during the early post-transplant phase can trigger rapid liver regeneration, it has also been associated with graft dysfunction [9]. While multiple strategies have been employed to address early allograft dysfunction in LDLT, practices such as graft selection to ensure a GRWR exceeding 0.8%, facilitating complete venous drainage, and adjusting inflow have now become established standards in the field of LDLT [22,23]. Additional studies are required to evaluate the association between small-for-size syndrome and post-transplant HCC recurrence [24].
This study had several limitations, including its retrospective nature and the enrollment of patients from a single center in a region with a high prevalence of hepatitis B virus infection. Additionally, most of the HCC cases included in this study originated in livers infected with hepatitis B virus. Only 4 patients had very low GRWR (<0.7%) and the median GRWR in the group with GRWR <0.8% was 0.75%. Therefore, the prognostic effect of very low GRWR could not be investigated in this study.
Conclusions
The present high-volume study demonstrated that GRWR <0.8% did not have a significant prognostic impact on the oncological outcomes of patients undergoing LT for HCC. It is necessary to perform further high-volume multi-center studies to validate the findings of the present study.
Figures
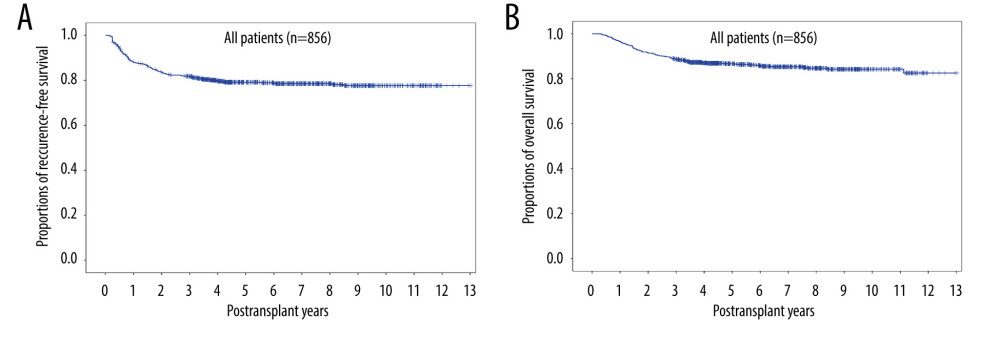 Figure 1. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in all 856 patients who underwent living donor liver transplantation for hepatocellular carcinoma.
Figure 1. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in all 856 patients who underwent living donor liver transplantation for hepatocellular carcinoma. 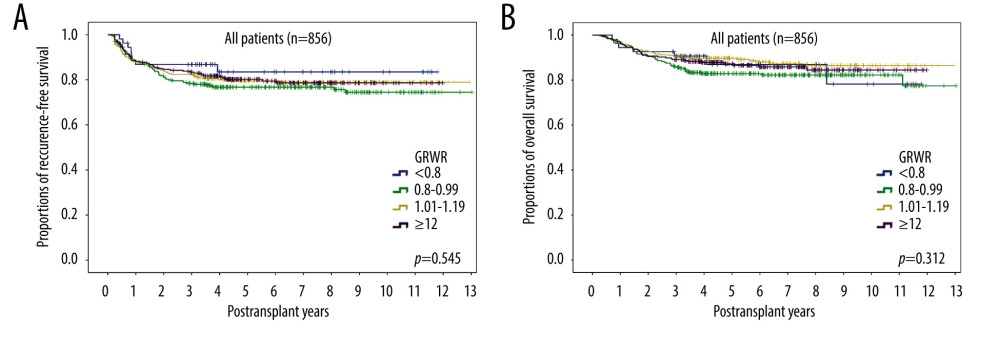 Figure 2. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in patients subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 2. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in patients subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. 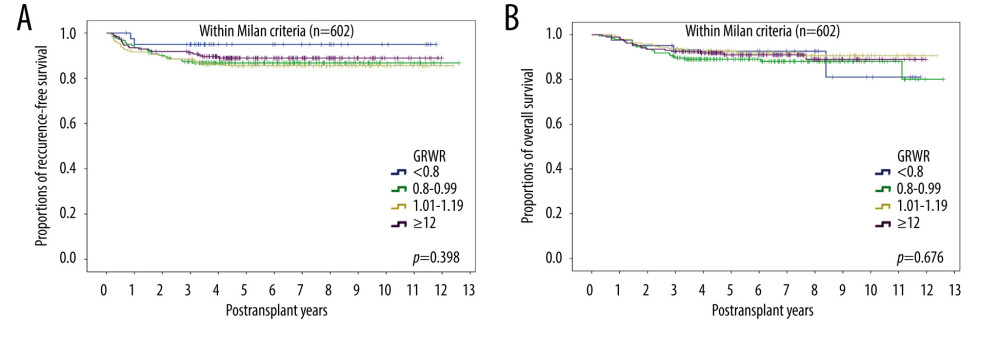 Figure 3. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 602 patients within the Milan criteria subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 3. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 602 patients within the Milan criteria subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. 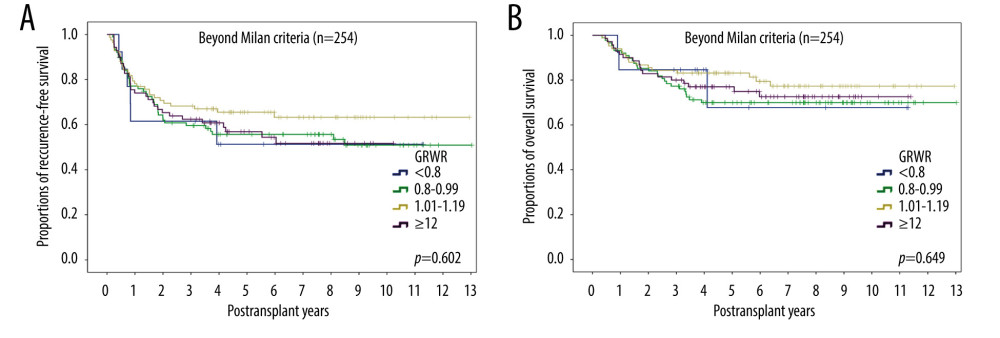 Figure 4. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 254 patients beyond the Milan criteria subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 4. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 254 patients beyond the Milan criteria subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. 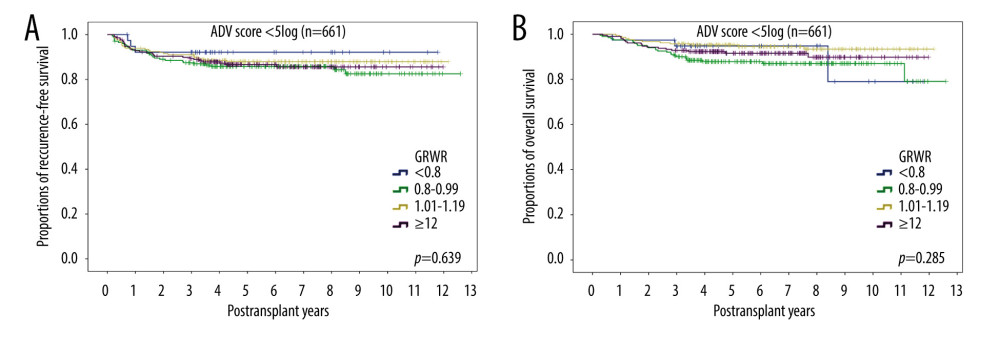 Figure 5. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 661 patients with ADV scores <5log subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 5. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 661 patients with ADV scores <5log subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. 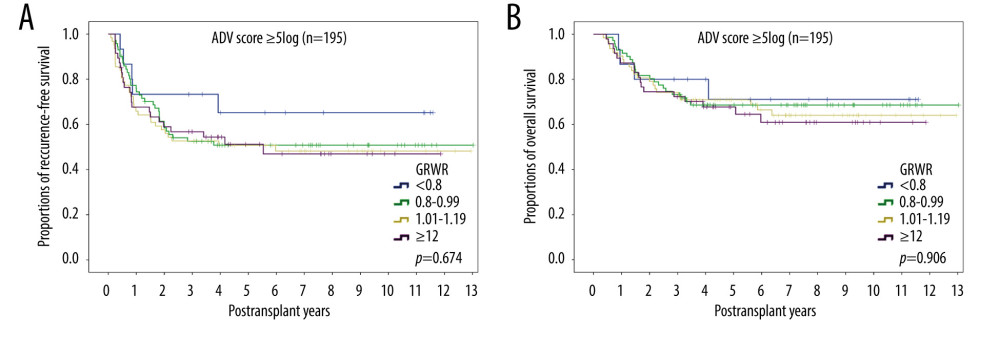 Figure 6. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 195 patients with ADV scores ≥5log subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 6. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 195 patients with ADV scores ≥5log subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. References
1. Fitzmaurice C, Allen C, Barber RMGlobal Burden of Disease Cancer Collaboration, Global, regional, and National Cancer Incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study: JAMA Oncol, 2017; 3; 524-48
2. Chan SC, Fan ST, Lo CM, A decade of right liver adult-to-adult living donor liver transplantation: the recipient mid-term outcomes: Ann Surg, 2008; 248; 411-49
3. Fisher RA, Kulik LM, Freise CE, Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation: Am J Transplant, 2007; 7; 1601-8
4. Chan SC, Section 2. Small-for-size liver graft and hepatocellular carcinoma recurrence: Transplantation, 2014; 97(Suppl 8); S7-S10
5. Lee EC, Kim SH, Shim JR, Park SJ, Small for size grafts increase recurrence of hepatocellular carcinoma in liver transplantation beyond Milan criteria: Liver Transpl, 2018; 24; 35-43
6. Hwang S, Lee SG, Ahn CS, Small-sized liver graft does not increase the risk of hepatocellular carcinoma recurrence after living donor liver transplantation: Transplant Proc, 2007; 39; 1526-29
7. Hu Z, Zhong X, Zhou J, Smaller grafts do not imply early recurrence in recipients transplanted for hepatocellular carcinoma: A Chinese experience: Sci Rep, 2016; 26; 26487
8. Man K, Lo CM, Xiao JW, The significance of acute phase small-for-size graft injury on tumor growth and invasiveness after liver transplantation: Ann Surg, 2008; 247; 1049-57
9. Yang ZF, Poon RT, Luo Y, Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway: J Immunol, 2004; 173; 2507-15
10. Picardo A, Karpoff HM, Ng B, Partial hepatectomy accelerates local tumor growth: Potential roles of local cytokine activation: Surgery, 1998; 124; 57-64
11. Hwang S, Song GW, Lee YJ, Multiplication of tumor volume by two tumor markers is a post-resection prognostic predictor for solitary hepatocellular carcinoma: J Gastrointest Surg, 2016; 20; 1807-20
12. Hwang S, Song GW, Ahn CS, Quantitative prognostic prediction using ADV score for hepatocellular carcinoma following living donor liver transplantation: J Gastrointest Surg, 2021; 25; 2503-15
13. Park GC, Hwang S, You YK, Quantitative prediction of posttransplant hepatocellular carcinoma prognosis using ADV Score: Validation with Korea-nationwide transplantation registry database: J Gastrointest Surg, 2023; 27; 1353-66
14. Leung JY, Zhu AX, Gordon FD, Liver transplantation outcomes for early-stage hepatocellular carcinoma: Results of a multicenter study: Liver Transpl, 2004; 10; 1343-54
15. Zavaglia C, De Carlis L, Alberti AB, Predictors of long-term survival after liver transplantation for hepatocellular carcinoma: Am J Gastroenterol, 2005; 100; 2708-16
16. Mazzaferro V, Regalia E, Doci R, Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis: N Engl J Med, 1996; 334; 693-99
17. Lee SG, Hwang S, Moon DB, Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center: Liver Transpl, 2008; 14; 935-45
18. Fujiki M, Takada Y, Ogura Y, Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma: Am J Transplant, 2009; 9; 2362-71
19. Mazzaferro V, Llovet JM, Miceli RMetroticket Investigator Study Group, Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis: Lancet Oncol, 2009; 10; 35-43
20. Kiuchi T, Tanaka K, Ito T, Small-for-size graft in living donor liver transplantation: How far should we go?: Liver Transpl, 2003; 9; S29-35
21. Yagi S, Iida T, Taniguchi K, Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation: Liver Transpl, 2005; 11; 68-75
22. Ikegami T, Kim JM, Jung DH, Conceptual changes in small-for-size graft and small-for-size syndrome in living donor liver transplantation: Korean J Transplant, 2019; 33; 65-73
23. Cheng P, Li Z, Fu Z, Small-for-size syndrome and graft inflow modulation techniques in liver transplantation: Dig Dis, 2023; 41; 250-58
24. Limkemann AJP, Abreu P, Sapisochin G, How far can we go with hepatocellular carcinoma in living donor liver transplantation?: Curr Opin Organ Transplant, 2019; 24; 644-50
Figures
 Figure 1. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in all 856 patients who underwent living donor liver transplantation for hepatocellular carcinoma.
Figure 1. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in all 856 patients who underwent living donor liver transplantation for hepatocellular carcinoma. Figure 2. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in patients subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 2. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in patients subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. Figure 3. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 602 patients within the Milan criteria subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 3. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 602 patients within the Milan criteria subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. Figure 4. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 254 patients beyond the Milan criteria subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 4. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 254 patients beyond the Milan criteria subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. Figure 5. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 661 patients with ADV scores <5log subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 5. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 661 patients with ADV scores <5log subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. Figure 6. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 195 patients with ADV scores ≥5log subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests.
Figure 6. Kaplan-Meier analyses of (A) disease-free survival and (B) overall survival in the 195 patients with ADV scores ≥5log subgrouped by graft-to-recipient weight ratio (GRWR). Comparisons by log-rank tests. Tables
In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860