30 July 2021: Original Paper
Clinical Outcomes of Ablation Compared with Resection for Single Hepatocellular Carcinoma Lesions, as a Primary Treatment or Bridging to Liver Transplantation: A Retrospective Comparative Study
Elizabeth M. Ward1ABCDEF, Ahmed Elshawadfy Sherif12BCDEF*, Stephen O'Neill3CD, Michael Hughes1B, Hamish Ireland4DE, Stephen J. Wigmore1ADE, Anya Adair1ADEDOI: 10.12659/AOT.931980
Ann Transplant 2021; 26:e931980
Abstract
BACKGROUND: Ablative therapies (AT) are widely utilized as bridging treatment for liver transplantation (LT) candidates with hepatocellular carcinoma (HCC) who are on the transplant waiting list to minimize dropout rate. We aimed to investigate whether AT could be considered a primary treatment modality for LT candidates with single, small HCC lesions.
MATERIAL AND METHODS: We retrospectively investigated the outcomes of patients with AT for single HCC lesions as primary treatment or bridging to LT between 2010 and 2017, compared with surgical resection (SR) during the same time period as control. Univariate and multivariate survival analyses were performed. Matched analysis, after propensity score matching (PSM), was performed to minimize the selection bias confounding effect on outcomes.
RESULTS: Of 162 patients identified, 92 received AT and 70 had SR. PSM identified 38 paired matches in each group. Overall survival (OS) and disease-free survival (DFS) before matching showed comparable outcomes for each treatment after 1, 3, and 5 years. Multivariate analysis using Cox regression models adjusting the study confounders showed lesion size (>30 mm), not treatment received, was associated with worse DFS (hazard ratio, 2.21 [95% confidence interval, 1.14-4.28]). In the matched groups, OS and DFS were equivalent and consistent with the whole-cohort survival outcomes. Explant histopathology of patients having AT as a bridge to LT showed complete pathological response in 85.7% of patients.
CONCLUSIONS: This study supports the use of AT with curative intent for single ≤3-cm HCCs, particularly in LT candidates, with salvage transplantation kept as a backup in case of recurrence.
Keywords: Ablation Techniques, Carcinoma, Hepatocellular, Hepatectomy, Liver Transplantation, Liver Neoplasms
Background
The increasing prevalence of cirrhosis and liver disease in Western populations due to alcohol consumption, non-alcoholic fatty liver disease, and hepatitis has in turn led to hepatocellular carcinoma (HCC) becoming the fifth most prevalent tumor worldwide and a major cause of death [1]. The incidence of this cancer is predicted to double over the next 2 decades, which carries with it a huge burden on healthcare systems [2].
The Barcelona Clinic Liver Cancer (BCLC) algorithm is widely accepted as a model to predict outcomes and guide the most suitable treatment in HCC in Western populations [3,4]. Patients with single, small HCC lesions (<30 mm), preserved liver function, and no portal hypertension can be considered for surgical resection in the absence of other significant comorbidities. Surgical resection is deemed curative but does carry the risk of recurrence at the resection margin and of developing new tumors in the remaining cirrhotic liver parenchyma. In these cases, salvage transplantation can be considered.
Liver transplantation (LT) is considered the criterion standard of care in the treatment of unresectable HCCs, and for those patients with Childs-Pugh B/C liver disease, because it provides a cure to the underlying disease in addition to tumor removal. To ensure acceptable outcomes in LT, criteria must be met when considering patient eligibility. The Milan criteria are the most widely used indications for LT in patients with HCC (1 nodule ≤5 cm or no more than 3 nodules ≤3 cm), and patients within these criteria have overall rates of survival comparable to those undergoing LT without HCC [5,6]. Other criteria have evolved to consider tumor biology, but only a few have been validated by multiple independent studies [7]. The use of the University of California, San Francisco criteria allows for transplantation in patients with larger tumors and considers tumor biology (a single lesion ≤6.5 cm, or 2–3 lesions ≤4.5 cm each, with total tumor diameter ≤8 cm) [8].
The shortage of available transplantable organs means demand is much greater than supply. Allocation systems tend to prioritize patients with severe chronic liver disease for LT, and, consequently, many patients with HCC with reasonably well-preserved liver function test are considered low priority for LT. Such patients can advance beyond transplant criteria owing to tumor progression while on the waiting list. The utilization of locoregional bridging therapies to minimize tumor progression and dropout from waiting lists is widely employed [9].
Ablative therapies can be used in the setting of transplantation as bridging treatment or as definitive management, where neither resection nor transplant is possible. Ablative therapies have been shown to demonstrate complete pathological response and improved long-term survival after transplantation when used as a bridging treatment [10–12]. Previous literature has generally shown higher recurrence rates with ablative therapies than with surgical resection [13–17]. However, ablation techniques have evolved over time, and the current widespread use of effective antivirals for hepatitis C is thought likely to reduce the incidence of new tumor formation in patients who have undergone ablative therapies or surgical resection. The efficacy of ablation for consideration as a primary treatment for small HCC lesions is not well established, with a low or very low quality of evidence in the literature assessing the long-term outcomes of ablative therapies as compared with surgical resection [18]. In practice, ablation is commonly employed as an alternative treatment in those patients for whom resection is inappropriate.
In this study, we investigated the outcomes of patients receiving ablation for single HCC lesions either as a primary treatment or as a bridge to transplantation in LT candidates. We directly compared outcomes of patients undergoing ablative therapies to those of patients undergoing surgical resection in our unit to assess the efficacy of ablation as a primary treatment option. The explant histology of patients who went on to receive LT following ablative therapy treatment were examined to assess the pathological response to ablative treatment. Our aim was to explore whether ablation can be considered as a definitive curative treatment modality, with the possibility of salvage transplantation in cases of recurrence.
Material and Methods
STUDY DESIGN AND SETTING:
All consecutive patients who had ablative therapies for single HCCs in a single-center tertiary hepato-pancreato-biliary and LT unit between January 2010 and January 2017 were identified from a retrospective review of prospectively collected data. Data for individuals who had surgical resection for single HCCs over the same period were gathered for direct comparison. Local Institutional Review Board and Caldicott Guardian consent to access patient data were granted. Patients’ electronic medical records were examined to review basic demographics, treatment received, multi-disciplinary team discussions, and radiological evidence of recurrence in each group. Information related to eligibility and assessment for LT and inclusion to the waiting list was recorded. In patients who received LT after ablative therapies, the histopathology reports of explant hepatectomies were retrieved from electronic records, and details regarding the ablative sites were collected for analysis. The end follow-up date for all patients involved in the study was January 2018. Follow-up for patients who received LT was censored, and the transplant date was marked as their last follow-up.
INCLUSION AND EXCLUSION CRITERIA:
All adult (>18 years old) male and female patients presenting with a single HCC lesion for which they received either ablative therapy or surgical resection were included. Individuals who received both ablative therapies and surgical resection within the study period were excluded from the analysis to avoid the mixed treatment effect on the outcomes.
MEASUREMENTS:
Our primary outcome measures included overall survival (OS), disease-free survival (DSF), and radiological evidence of local recurrence at the treatment site. The secondary outcomes were complication rates and the pathological response to ablative therapies in the explant hepatectomy for patients who received LT.
TREATMENT PROTOCOL:
All included patients were discussed at the local multi-disciplinary team meeting where ablative therapies, surgical resection, or assessment for transplantation were recommended. The decision making that guided which treatment was appropriate was performed in a case by case discussion among surgeons, radiologists, hepatologists, and oncologists and was based on the patients’ performance status, degree of preserved liver function tests, and tumor number, size, and location in the liver.
ABLATIVE THERAPIES:
Patients suitable for percutaneous ablation were selected at the local hepatobiliary multi-disciplinary meeting. Ablations were performed percutaneously under general anesthesia using ultrasound or computerized tomography (CT) guidance by a single consultant radiologist (HI). Artificial ascites was used for higher-risk peripheral tumors. A monopolar multi-tined 480-kHz radiofrequency ablation system (Boston RF3000, Boston Scientific Corporation, Natick, MA, USA) was used. For later patients, particularly those with lesions >3 cm, we used a 2.45-GHz microwave ablation system (Acculis, Angiodynamics, Latham, NY, USA).
Follow-up imaging consisted of a dual-phase CT (arterial and portal venous phase) at 1 month and then dual-phase CT at 4-month intervals, with the addition of magnetic resonance imaging (MRI) of the liver for equivocal cases. Treatment response was assessed by imaging to evaluate the ablative zone as well as any evidence of locoregional or distant disease.
SURGICAL RESECTION:
Surgical resections were performed in a single tertiary referral center using a mixture of laparoscopic and open surgical procedures. Intraoperative ultrasound was routinely performed to allow localization of the tumor, provide an accurate vascular map of feeding vessels, and establish general liver anatomy.
Resection of the liver parenchyma was done using a Cavitron ultrasonic surgical aspirator, often in combination with a Thunderbeat (Olympus Medical Systems) or Ultracision (Ethicon Endosurgery) device. Hemostasis was achieved using a combination of bipolar coagulation, titanium vascular clips, tying or suturing, and an argon beam coagulator. Postoperatively, patients were managed in a high-dependency unit before being transferred to a general surgical ward. Patients undergoing surgical resection followed local protocol, with follow-up imaging in the form of MRI every 4 months during the first year and every 6 months during the second year, followed by ultrasound imaging thereafter.
LIVER TRANSPLANTATION:
Patients were considered for listing for LT based on the current United Kingdom LT listing criteria for HCC [19].
STATISTICAL ANALYSIS:
Numerical parametric data are presented as mean±standard deviation and non-parametric data as median and interquartile range. Categorical data are presented as frequency and percentage. Statistical comparisons of unpaired continuous parametric data and non-parametric data were performed using the
Results
SURVIVAL ANALYSIS:
The univariate analysis results of OS and DFS before PSM are shown in Figure 2. There were no statistically significant differences in OS and DFS between the AT group and SR group at 1, 3, and 5 years after treatment according to the log-rank test.
In the unmatched cohort, multivariate analysis was performed using Cox regression survival models for OS and DFS to investigate the adjusted treatment effect on the outcomes in the larger study sample before matching. As shown in Table 2, the results indicated that ablative therapies and surgical resection had comparable treatment effects on OS and DFS, while HCC lesion size appeared to have a significant impact on both models.
Since HCC lesion size in both treatment groups appeared to have an impact on OS and DFS, Cox regression models were performed by dividing the lesion size into 2 groups: 0 to 30 mm and >30 mm, corresponding to BCLC stage 0/A and beyond stage A, respectively. The results shown in Table 2 and Figure 3 demonstrate that DFS appeared to have been significantly lower in patients with HCC lesions >30 mm in size (hazard ratio, 2.21 [95% confidence interval, 1.14–4.28]). However, the treatment modality they received (ablative therapy or surgical resection) did not have any significant impact on OS and DFS.
After PSM, OS and DFS were compared between the 2 groups (Figure 2). The results were consistent with the univariate and multivariate analyses of the whole cohort, and did not show any statistically significant difference in OS and DFS between ablative therapies and surgical resection at 1, 3, and 5 years after treatment.
THE PATTERN OF HCC RECURRENCE AFTER ABLATIVE THERAPIES AND SURGICAL RESECTION:
The pattern of HCC recurrence in the whole cohort is shown in Figure 4 and Table 3. The data suggested there were significant differences between the groups of patients who had no recurrence and those who had all other patterns of recurrence. Kaplan-Meier survival analysis showed that extrahepatic recurrence was significantly higher in the SR group, while local and de novo hepatic recurrence was comparable between the 2 groups. We explored the tumor characteristics among the different patterns of recurrence with ablative therapies and surgical resection (Table 4). HCC lesion size, serum alpha-fetoprotein, tumor involvement at the resection margin, microvascular invasion, and tumor differentiation in the SR group did not appear to have a statistically significant pattern to explain the higher extrahepatic metastasis pattern.
The extent of recurrence differed between the 2 groups: the AT group had a higher proportion of patients developing recurrence within the UK transplant listing criteria, compared with patients in the SR group. Among patients who had received ablative therapies and surgical resection as a primary treatment, 3 patients had tumor recurrence that were within transplant criteria (range, 12.9–18.6 months), and they successfully received salvage LT with good long-term survival outcomes (1 patient in the AT group and 2 patients in the SR group).
ANALYSIS OF LT EXPLANT HISTOPATHOLOGY:
When examining the explant histopathology of patients undergoing ablative therapies who went on to receive a transplant, a total of 14 patients were identified. The mean patient age was 59.5 years (range, 49–74 years), and the mean UKELD score was 48 (range, 43.4–53.7). Ablated lesions were a mean of 19 mm (range, 9–28 mm) in size. Complete pathological response to ablation (complete necrosis with no evidence of microvascular invasion) in the explants was noted in 12 patients (85.7%), and evidence of viable tumor was seen in 2 patients (14.3%). The average timing between ablative therapies and transplantation was 7.1 months (range, 0.3–20.7 months). One patient with a viable tumor received a transplant only 13 days after ablative treatment, and despite the short interval, the ablated lesion showed an area of 60% necrosis. The second patient was 15.1 months after ablative treatment at the time of LT, and the histopathology showed a small nodule of viable HCC at the periphery of the ablation burn zone; this was not visible on previous follow-up imaging, with CT scanning demonstrating a good ablation zone.
Discussion
This study investigated using ablative therapies for single, small HCC lesions as a primary treatment or as a bridge to transplantation in LT candidates in comparison with surgical resection. The findings showed that since ablative therapies had survival benefits comparable to surgical resection, ablative treatment could be offered as an alternative primary treatment for patients with single HCC lesions ≤3 cm in size, particularly in LT candidates with less preserved liver function tests that were not amenable for surgical resection. Our results also revealed that the extrahepatic pattern of recurrence in patients who received ablative therapies was significantly lower than that of surgical resection, which could help give those patients better chances of being considered for salvage transplantation if local recurrence develops.
The 2 groups of the main cohort in our study were matched by age and sex. There was a significant difference in UKELD score between the groups, which can be explained by the local treatment policy for small lesions: patients with preserved liver function typically undergo surgical resection, while ablative therapies are reserved for those patients with poorer liver function, owing to the increased morbidity of surgery for these patients. A significant difference was also observed in HCC lesion size, with larger lesions seen in the SR group. This was expected because the use of radiofrequency ablation is technically limited to lesions less than 3 cm in size to achieve good results. The introduction of microwave ablation has allowed an increase in the size of lesions being ablated [20], which was reported to have equivalent efficacy and survival when compared with radiofrequency ablation [21,22]. When PSM was applied to the main cohort, HCC lesion size and UKELD score in the 2 groups were well matched.
When comparing OS and DFS before and after PSM, our results demonstrated comparable rates in ablative therapies and surgical resection at 1, 3, and 5 years after treatment. A multivariate analysis using a Cox regression model was performed in the unmatched cohort to adjust for HCC lesion size and patients’ UKELD scores as potential confounders. This showed that ablative therapies had a nonsignificant lower treatment effect on OS and DFS, while lesion size appeared to have a significant effect on both OS and DFS models. This suggested that lesion size, and not the treatment modality performed, influenced survival.
We specifically explored the lesion-size effect by performing a subgroup analysis classifying lesion size into 2 groups, 0 to 30 mm and >30 mm, to correspond to BCLC stage 0/A and beyond stage A, respectively [4]. Using a multivariate Cox regression model, lesions sized 0 to 30 mm appeared to be the size that achieved the best outcomes for ablative therapies, and lesions larger than 30 mm had a significant negative effect on DFS.
Our findings support other studies that included patient cohorts from Western populations with predominantly nonviral HCC disease [23,24]. In a large retrospective study in a Western population including 544 patients, PSM demonstrated higher rates of local tumor progression with radiofrequency ablation but comparable OS and DFS results to surgical resection [25]. Their study was somewhat limited because of heterogeneity since it included results from 15 different centers and cases were from 1999 to 2010, a time period since which the ablative therapy techniques have rapidly developed.
Previous literature reporting favorable outcomes in surgical resection included predominantly younger non-Western patient populations, in which hepatitis B is the most common etiology for HCC [14,16,17,26–28]. Additionally, the OS of patients with HCC is determined by underlying liver function, with the general understanding that patients with good function are selected for surgical resection and those with poorer function undergo ablative therapies [6]. Many of these studies were conducted in centers where liver function between the 2 groups is more favorable to the resection groups, thereby influencing OS and DFS rates [27].
Where randomized controlled trials have looked specifically at surgical resection vs ablation, the results of meta-analyses and systematic reviews have concluded that evidence is of a low level and quality [18,29]. Unfortunately, heavy influence from the background demographic area exists, creating large variability between studies. Generally, the proportion of patients demonstrating local recurrence in the liver is shown to be lower in the surgical groups, but with an increased number of adverse events in this group when compared to those undergoing ablative therapies [29]. The decreased length of hospital stay and cost-effectiveness of ablative therapies is supported across all the literature [4,18].
It is undeniable that surgical resection does hold some advantages by removing a tumor, particularly if the HCC lesions are large. However, in populations with a higher prevalence of cirrhosis, surgical risks for mortality and morbidity increase significantly. The mortality and morbidity in our surgical cohort was equivalent to that of other studies [18].
Our results showed that overall HCC recurrence in the AT group was significantly lower than that in the SR group. Interestingly, the pattern of recurrence showed that local recurrence rates were higher in the AT group, yet distant extrahepatic recurrence was significantly lower in the SR group. This shows that the recurrences following ablative therapies are more likely to be within transplant criteria, which could allow more opportunity for salvage transplantation if ablative therapies are used as a primary treatment modality. Our study was not designed or adequately powered to explore why the SR group had a higher incidence of extrahepatic recurrence than did the AT group. This could be attributed to the treatment effect, but we also acknowledge that other technical and tumor biology confounders might have contributed. Our analysis of the correlation of tumor characteristics with the pattern of recurrence, particularly in the SR group, which did not show statistically significant associations, should be interpreted with care because of the very small number of events in some of the subgroups. Nevertheless, our results showed a better pattern of recurrence with ablative therapies, compared with previous studies reported in the literature [30,31].
When specifically assessing the explant histopathology of patients who had ablative therapies as a bridge to transplantation, the reports were favorable, confirming that complete pathological response had been achieved in 85.7% of patients. In 1 of the 2 patients in whom a viable tumor was seen, the time between ablative treatment and transplantation was so short that we concluded it would be too early to determine the treatment effect, although another patient did show complete response at 10 days after treatment of a 23-mm lesion. The second case showed a small microscopic satellite nodule not visible on radiological imaging to the ablated site more than 14 months later; whether this represented a new nodule or residual disease was unclear. Few studies have looked at explant histopathology, and those that have reported it date back to treatment from over 15 years ago, to a time when the techniques and use of ablative therapies were more immature, and complete necrosis was reported at around 70% [13,32]. The improved sophistication of the technique with microwave ablation now allows greater volume of necrosis and more effective treatment at previously anatomically difficult to reach lesions [21,22,33]. Nevertheless, it is also worth considering that there may be favorable bias in the tumor biology of individuals who went on to have LT because they made it to transplantation, rather than their disease progressing to such an extent that they no longer met the criteria of the waiting list.
Our study is limited by its retrospective nature, which introduced characteristic-selection bias. Nevertheless, performing a randomized controlled trial of ablative therapies and surgical resection still creates bias in an attempt to minimize the impact of confounding factors. Only patients meeting predefined characteristics can be randomized, thus excluding a proportion of patients who would ordinarily be treated in clinical practice.
We acknowledge that the results of the PSM analysis are underpowered owing to the relatively small number of matched pairs. Still, since the results of the matched analysis agreed with the unmatched univariate and multivariate analyses outcomes, this could provide a further degree of support of our findings by eliminating the confounding effect of selection bias on treatment outcomes.
Our patient population was representative of a Western population with the most common etiologies for HCC being hepatitis C, alcoholic liver disease, and non-alcoholic fatty liver disease. Our sample size does not carry the power of other studies but has the advantage of displaying the results of a single center, with a single interventional radiologist performing the ablative therapies, reducing treatment bias. Our period of patient selection also included more up-to-date techniques than those of previous studies [13,23,24,26].
Our findings support the use of ablation as a viable primary treatment modality in LT candidates with HCC, and not just as a bridging treatment to enhance patient allocation priority on the transplant waiting list. In cases of recurrence following AT, salvage transplantation should be considered. With increasing demand on healthcare worldwide as the prevalence of HCC grows, our study demonstrates ablative therapies as a safe, cost-effective alternative with a comparative survival benefit to surgical resection. Ablative therapies have well-documented advantages over resection, with lower mortality and morbidity, reduced length of hospital stay, and favorable surgical implications, if patients do go on to receive a transplant. In patients with cirrhosis, the risk of hepatic deterioration over time due to the loss of liver parenchyma seen with resection is also reduced [9].
Ablative therapies are beneficial to patients with severe portal hypertension, which is a negative prognostic indicator for surgical resection [7,9,12].
Increased utilization of ablative therapies for small, single HCC lesions as a primary treatment permits a reduction in healthcare burden as well as the saving of grafts, which can then be more efficaciously transplanted in other patients. The increased utilization of ablative therapies in these cases is also supported by the discrepancy between the limited donor pool and the enormous number of LT candidates.
Conclusions
Our study supports the increased use of ablative therapies with curative intent, with comparable OS and DFS rates to surgical resection in the settings of similar patients. Ultimately, the choice between the 2 treatments should be tailored to the individual patient through discussions between the surgical and radiological teams.
Figures
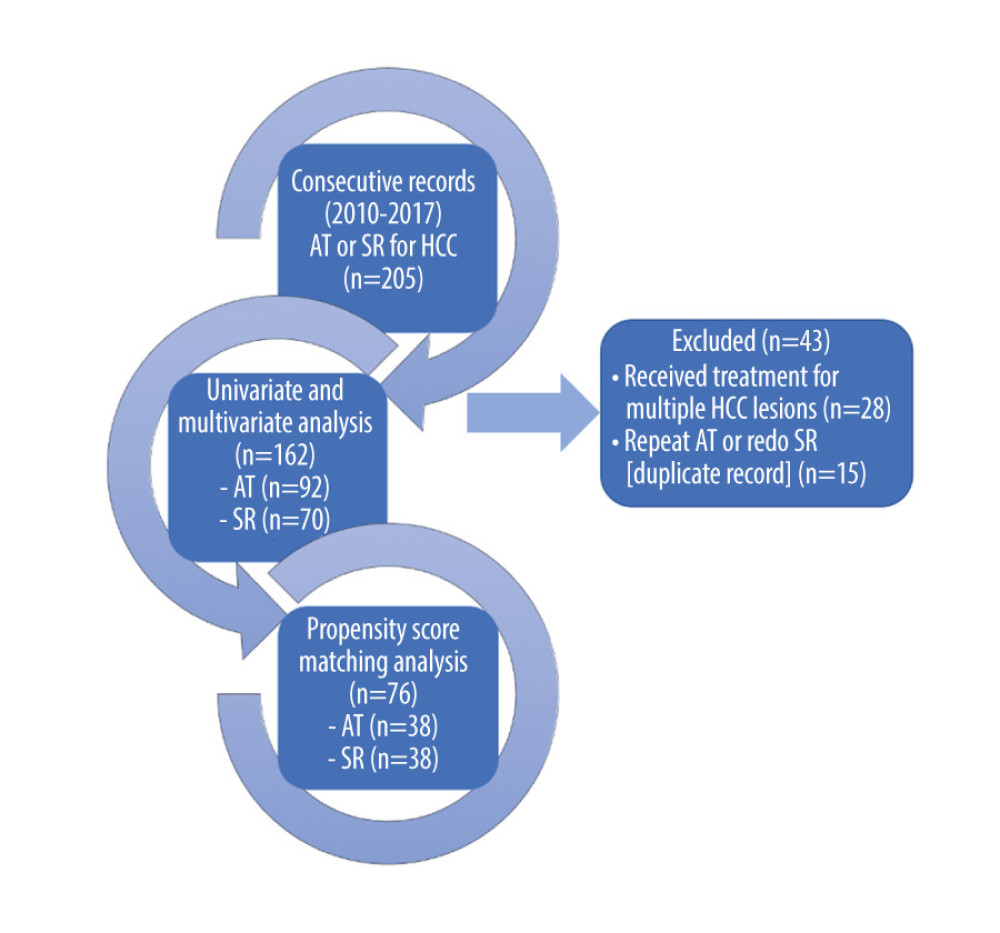 Figure 1. A flow diagram demonstrating patient selection for the study. AT – ablative therapies; HCC – hepatocellular carcinoma; SR – surgical resection
Figure 1. A flow diagram demonstrating patient selection for the study. AT – ablative therapies; HCC – hepatocellular carcinoma; SR – surgical resection 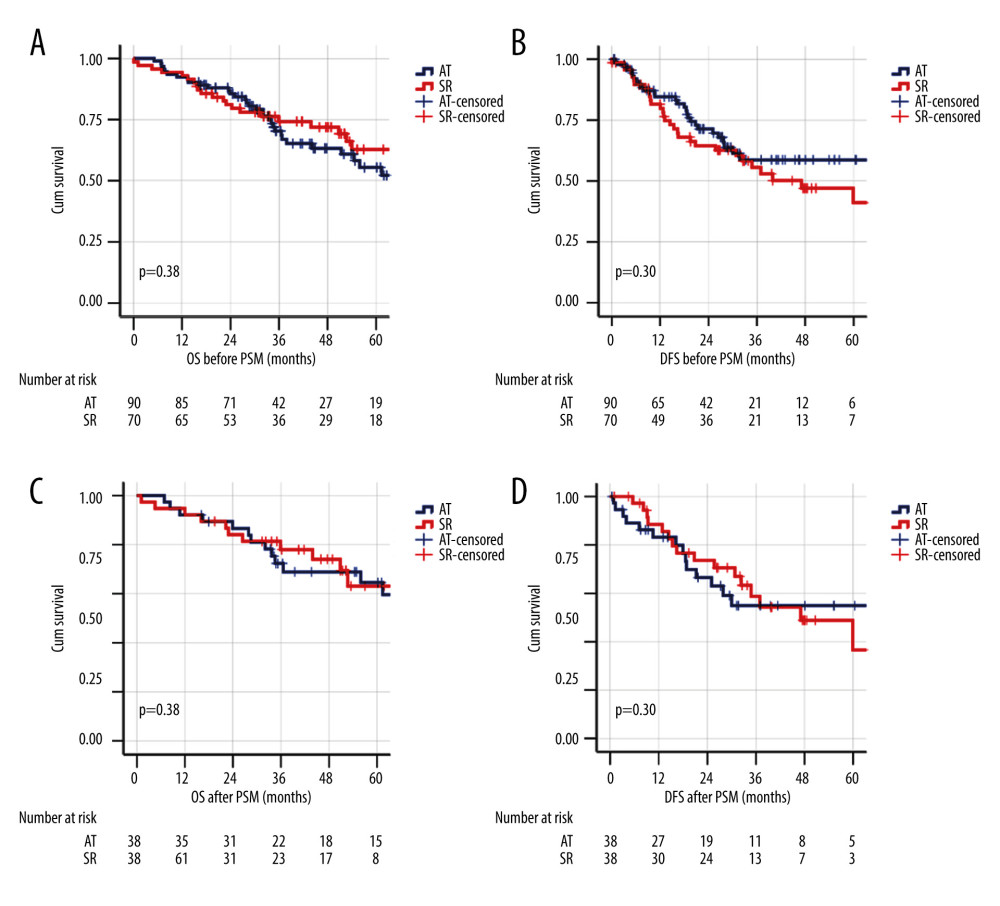 Figure 2. Kaplan-Meier survival analysis for overall survival (OS) and disease-free survival (DFS) in ablative therapies and surgical resection treatment groups before and after propensity score matching. (A) OS before matching. (B) DFS before matching. (C) OS after matching. (D) DFS after matching.
Figure 2. Kaplan-Meier survival analysis for overall survival (OS) and disease-free survival (DFS) in ablative therapies and surgical resection treatment groups before and after propensity score matching. (A) OS before matching. (B) DFS before matching. (C) OS after matching. (D) DFS after matching. 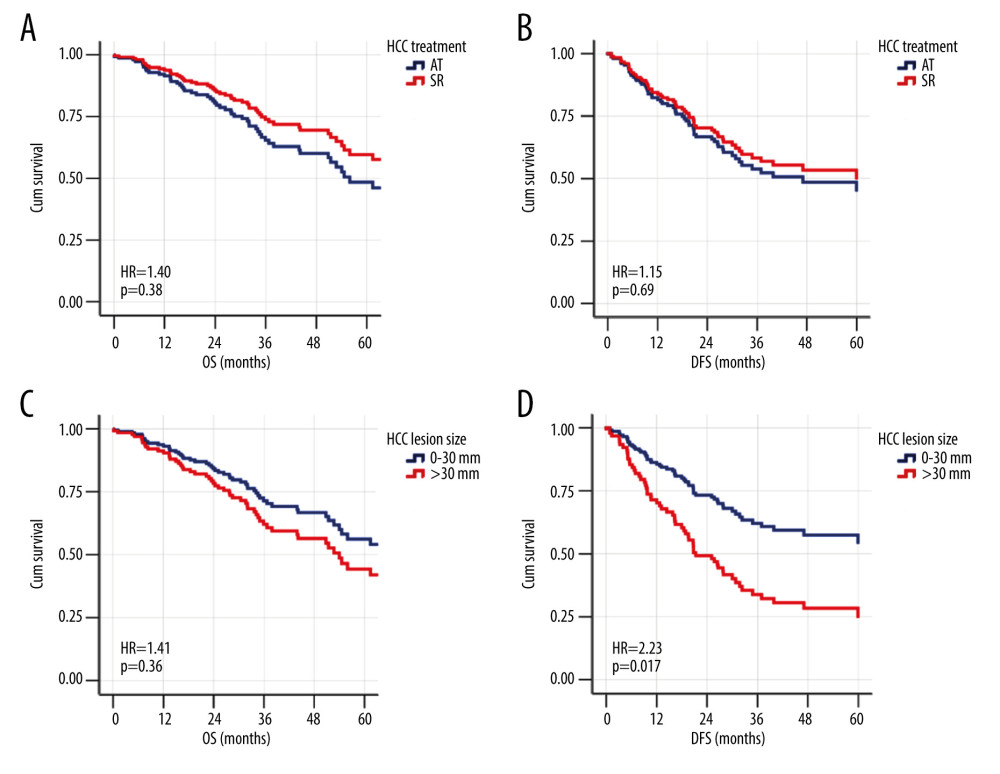 Figure 3. The adjusted effect of HCC treatment modality and lesion size on the patterns of overall survival (OS) and disease-free survival (DFS) of the whole cohort using Cox regression models. (A) Ablative therapy treatment effect on OS. (B) Ablative therapy treatment effect on DFS. (C) Effect of hepatocellular carcinoma (HCC) lesion size on OS. (D) Effect of HCC lesion size on DFS.
Figure 3. The adjusted effect of HCC treatment modality and lesion size on the patterns of overall survival (OS) and disease-free survival (DFS) of the whole cohort using Cox regression models. (A) Ablative therapy treatment effect on OS. (B) Ablative therapy treatment effect on DFS. (C) Effect of hepatocellular carcinoma (HCC) lesion size on OS. (D) Effect of HCC lesion size on DFS. 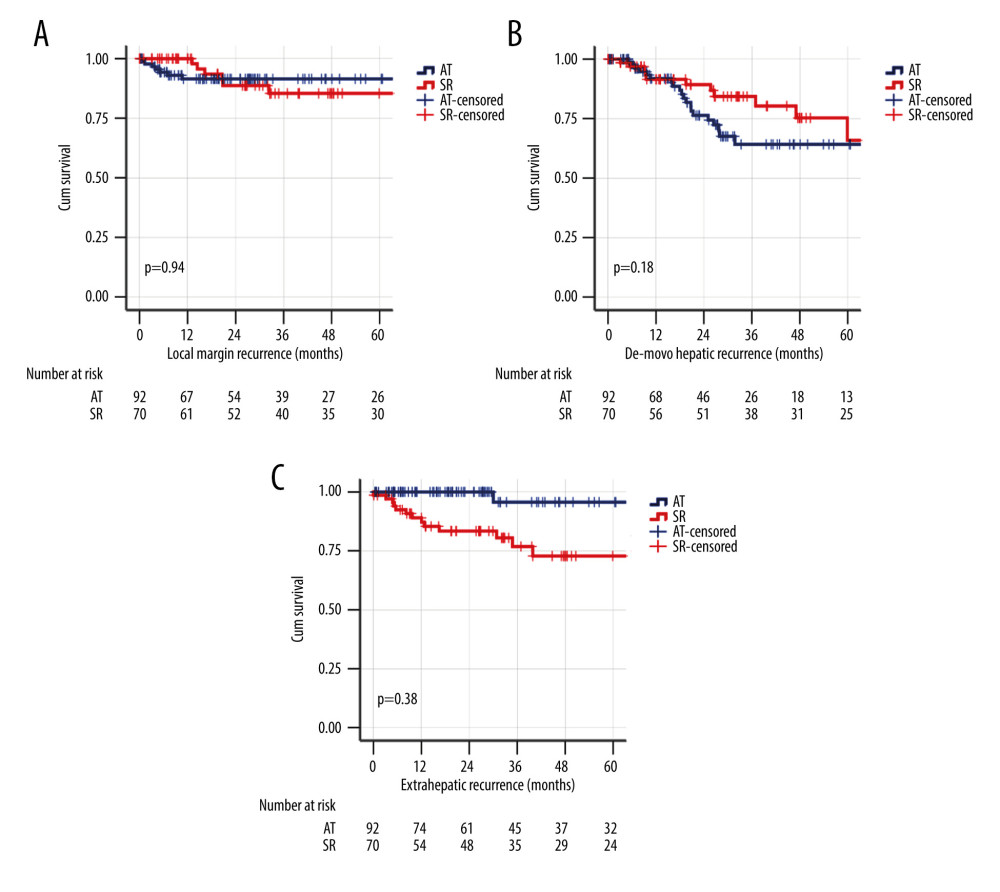 Figure 4. Kaplan-Meier survival analysis for different patterns of HCC recurrence in the whole cohort. (A) Local margin recurrence. (B) De novo hepatic recurrence. (C) Extrahepatic recurrence.
Figure 4. Kaplan-Meier survival analysis for different patterns of HCC recurrence in the whole cohort. (A) Local margin recurrence. (B) De novo hepatic recurrence. (C) Extrahepatic recurrence. Tables
Table 1. Characteristics of the study groups before and after matching.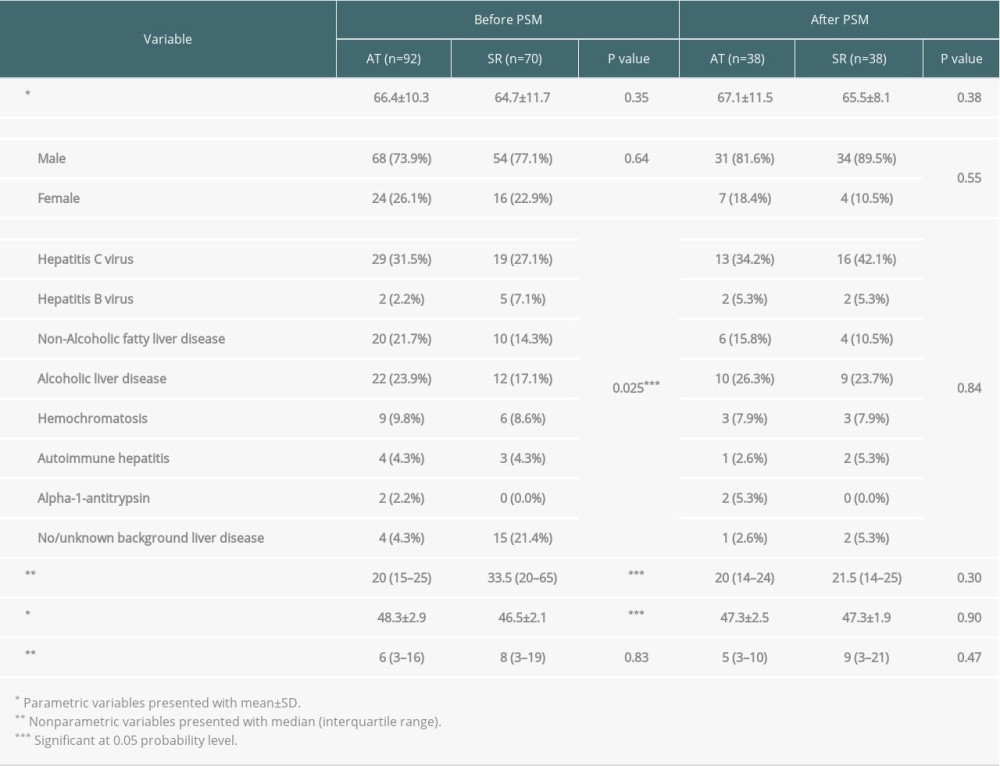 Table 2. Multivariate analysis using Cox regression models for overall survival and disease-free survival, adjusting for potential confounders in the study groups before matching.
Table 2. Multivariate analysis using Cox regression models for overall survival and disease-free survival, adjusting for potential confounders in the study groups before matching.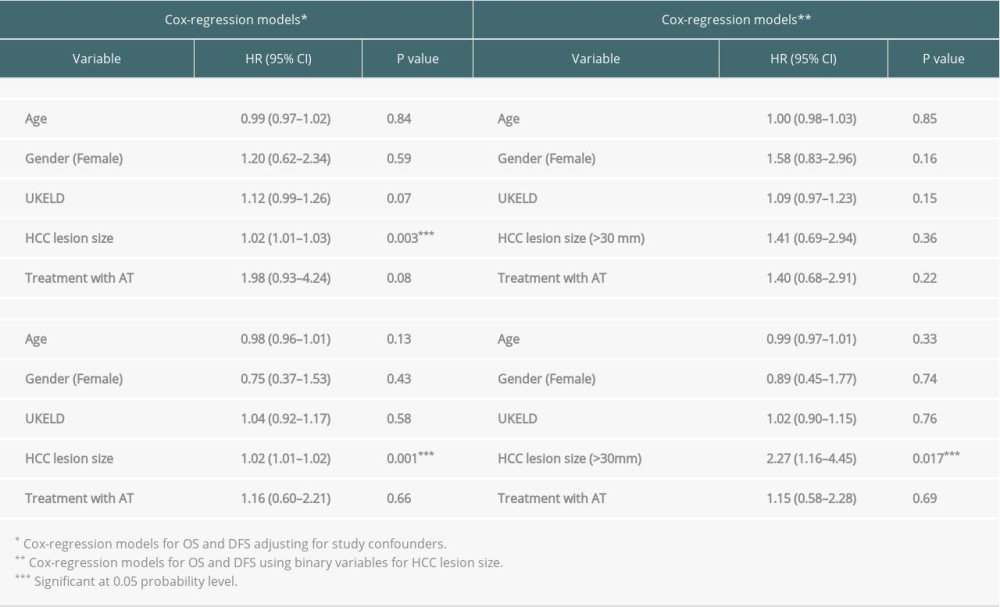 Table 3. The pattern of hepatocellular carcinoma recurrence in all patients after ablative therapies and surgical resection.
Table 3. The pattern of hepatocellular carcinoma recurrence in all patients after ablative therapies and surgical resection.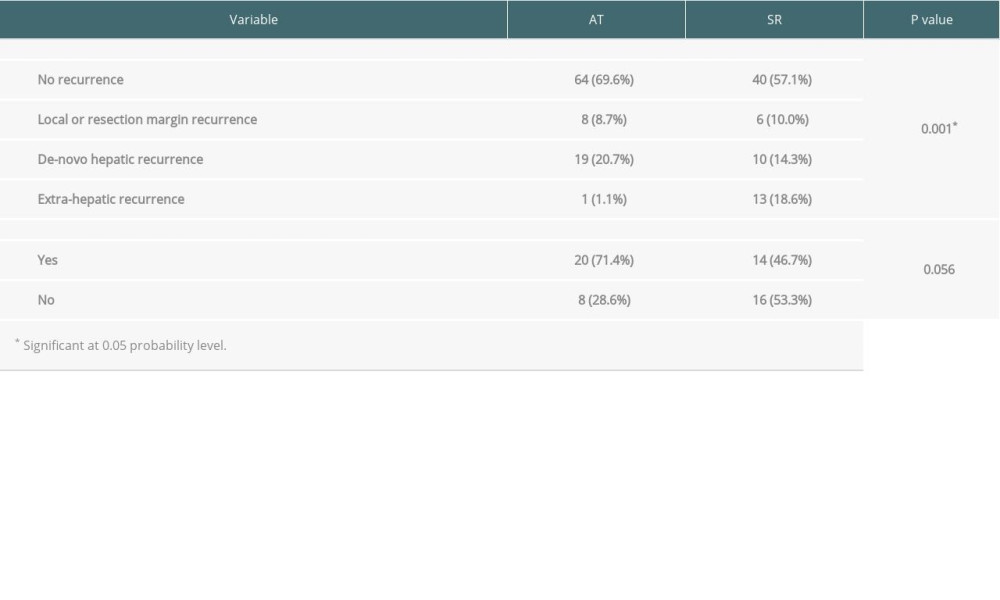 Table 4. Pattern of recurrence and tumor characteristics with ablative therapies and surgical resection.
Table 4. Pattern of recurrence and tumor characteristics with ablative therapies and surgical resection.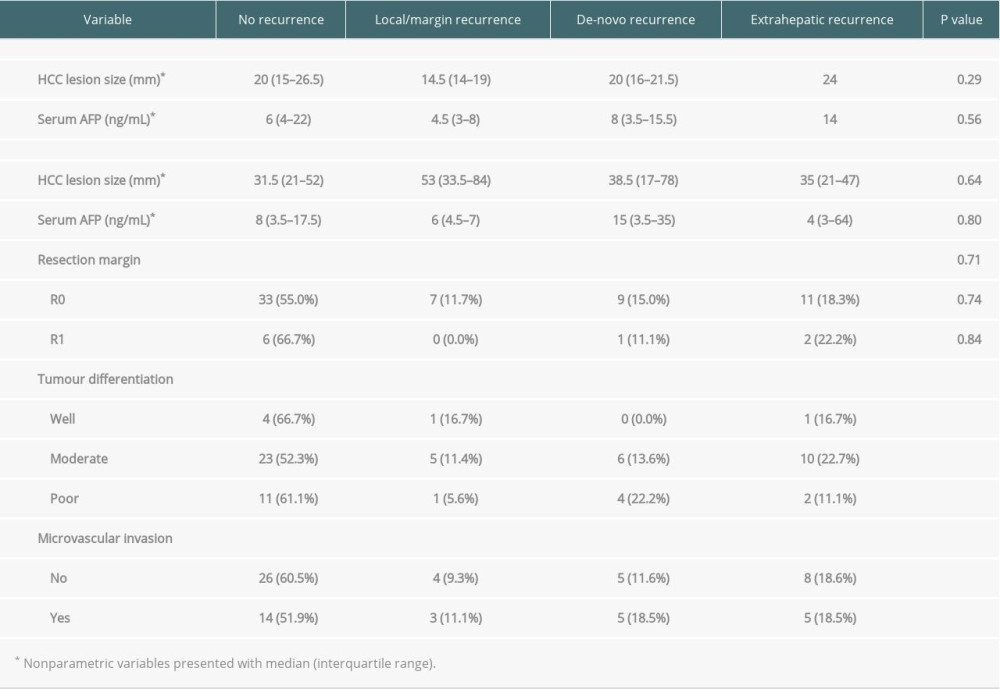
References
1. Balogh J, Victor D, Asham EH, Hepatocellular carcinoma: A review: J Hepatocell Carcinoma, 2016; 3; 41-53
2. Ghouri YA, Mian I, Rowe JH, Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis: J Carcinog, 2017; 16; 1
3. Llovet JM, Bru C, Bruix J, Prognosis of hepatocellular carcinoma: The BCLC staging classification: Semin Liver Dis, 1999; 19(3); 329-38
4. European Association for the Study of the Liver, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma: J Hepatol, 2018; 69(1); 182-236
5. Mazzaferro V, Regalia E, Doci R, Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis: N Engl J Med, 1996; 334(11); 693-99
6. Kulik L, Heimbach JK, Zaiem F, Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis: Hepatology, 2018; 67(1); 381-400
7. Elshamy M, Aucejo F, Menon KV, Eghtesad B, Hepatocellular carcinoma beyond Milan criteria: Management and transplant selection criteria: World J Hepatol, 2016; 8(21); 874-80
8. Yao FY, Ferrell L, Bass NM, Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival: Hepatology, 2001; 33(6); 1394-403
9. Park JW, Chen M, Colombo M, Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study: Liver Int, 2015; 35(9); 2155-66
10. Ng KK, Lam CM, Poon RT, Thermal ablative therapy for malignant liver tumors: A critical appraisal: J Gastroenterol Hepatol, 2003; 18(6); 616-29
11. Curley SA, Izzo F, Ellis LM, Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis: Ann Surg, 2000; 232(3); 381-91
12. Agopian VG, Morshedi MM, McWilliams J, Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: Analysis of 501 consecutively treated patients: Ann Surg, 2015; 262(3); 536-45
13. Lu DS, Yu NC, Raman SS, Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation: Hepatology, 2005; 41(5); 1130-37
14. Lee S, Kang TW, Cha DI, Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: Propensity score analyzes of long-term outcomes: J Hepatol, 2018; 69(1); 70-78
15. Cucchetti A, Mazzaferro V, Pinna AD, Average treatment effect of hepatic resection versus locoregional therapies for hepatocellular carcinoma: Br J Surg, 2017; 104(12); 1704-12
16. Chen MS, Li JQ, Zheng Y, A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma: Ann Surg, 2006; 243(3); 321-28
17. Huang J, Yan L, Cheng Z, A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria: Ann Surg, 2010; 252(6); 903-12
18. Majumdar A, Roccarina D, Thorburn D, Management of people with early- or very early-stage hepatocellular carcinoma: An attempted network meta-analysis: Cochrane Database Syst Rev, 2017; 3; CD011650
19. Zalewska K: NHSBT Liver Transplantation Selection Criteria and Recipient Registration [NHSBT Guidelines[, 2018 https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/9440/pol195_7-liver-selection-policy.pdf
20. Poggi G, Tosoratti N, Montagna B, Picchi C, Microwave ablation of hepatocellular carcinoma: World J Hepatol, 2015; 7(25); 2578-89
21. Glassberg MB, Ghosh S, Clymer JW, Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis: Onco Targets Ther, 2019; 12; 6407-38
22. Chong CCN, Lee KF, Cheung SYS, Prospective double-blinded randomized controlled trial of Microwave versus RadioFrequency Ablation for hepatocellular carcinoma (McRFA trial): HPB (Oxford), 2020; 22(8); 1121-27
23. Hocquelet A, Balageas P, Laurent C, Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: A study of 281 Western patients: Int J Hyperthermia, 2015; 31(7); 749-57
24. Pompili M, Francica G, Ponziani FR, Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation: World J Gastroenterol, 2013; 19(43); 7515-30
25. Pompili M, Saviano A, de Matthaeis N, Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey: J Hepatol, 2013; 59(1); 89-97
26. Kim GA, Shim JH, Kim MJ, Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas: Br J Surg, 2016; 103(1); 126-35
27. Ng KKC, Chok KSH, Chan ACY, Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma: Br J Surg, 2017; 104(13); 1775-84
28. Kim TH, Chang JM, Um SH, Comparison of 2 curative treatment options for very early hepatocellular carcinoma: Efficacy, recurrence pattern, and retreatment: Medicine (Baltimore), 2019; 98(26); e16279
29. Qi X, Zhao Y, Li H, Guo X, Han G, Management of hepatocellular carcinoma: An overview of major findings from meta-analyzes: Oncotarget, 2016; 7(23); 34703-51
30. Ng KK, Poon RT, Lo CM, Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma: J Gastrointest Surg, 2008; 12(1); 183-91
31. Lee SY, Konstantinidis IT, Eaton AA, Predicting recurrence patterns after resection of hepatocellular cancer: HPB (Oxford), 2014; 16(10); 943-53
32. Pompili M, Mirante VG, Rondinara G, Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence: Liver Transpl, 2005; 11(9); 1117-26
33. Lu MD, Xu HX, Xie XY, Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study: J Gastroenterol, 2005; 40(11); 1054-60
Figures
 Figure 1. A flow diagram demonstrating patient selection for the study. AT – ablative therapies; HCC – hepatocellular carcinoma; SR – surgical resection
Figure 1. A flow diagram demonstrating patient selection for the study. AT – ablative therapies; HCC – hepatocellular carcinoma; SR – surgical resection Figure 2. Kaplan-Meier survival analysis for overall survival (OS) and disease-free survival (DFS) in ablative therapies and surgical resection treatment groups before and after propensity score matching. (A) OS before matching. (B) DFS before matching. (C) OS after matching. (D) DFS after matching.
Figure 2. Kaplan-Meier survival analysis for overall survival (OS) and disease-free survival (DFS) in ablative therapies and surgical resection treatment groups before and after propensity score matching. (A) OS before matching. (B) DFS before matching. (C) OS after matching. (D) DFS after matching. Figure 3. The adjusted effect of HCC treatment modality and lesion size on the patterns of overall survival (OS) and disease-free survival (DFS) of the whole cohort using Cox regression models. (A) Ablative therapy treatment effect on OS. (B) Ablative therapy treatment effect on DFS. (C) Effect of hepatocellular carcinoma (HCC) lesion size on OS. (D) Effect of HCC lesion size on DFS.
Figure 3. The adjusted effect of HCC treatment modality and lesion size on the patterns of overall survival (OS) and disease-free survival (DFS) of the whole cohort using Cox regression models. (A) Ablative therapy treatment effect on OS. (B) Ablative therapy treatment effect on DFS. (C) Effect of hepatocellular carcinoma (HCC) lesion size on OS. (D) Effect of HCC lesion size on DFS. Figure 4. Kaplan-Meier survival analysis for different patterns of HCC recurrence in the whole cohort. (A) Local margin recurrence. (B) De novo hepatic recurrence. (C) Extrahepatic recurrence.
Figure 4. Kaplan-Meier survival analysis for different patterns of HCC recurrence in the whole cohort. (A) Local margin recurrence. (B) De novo hepatic recurrence. (C) Extrahepatic recurrence. Tables
 Table 1. Characteristics of the study groups before and after matching.
Table 1. Characteristics of the study groups before and after matching. Table 2. Multivariate analysis using Cox regression models for overall survival and disease-free survival, adjusting for potential confounders in the study groups before matching.
Table 2. Multivariate analysis using Cox regression models for overall survival and disease-free survival, adjusting for potential confounders in the study groups before matching. Table 3. The pattern of hepatocellular carcinoma recurrence in all patients after ablative therapies and surgical resection.
Table 3. The pattern of hepatocellular carcinoma recurrence in all patients after ablative therapies and surgical resection. Table 4. Pattern of recurrence and tumor characteristics with ablative therapies and surgical resection.
Table 4. Pattern of recurrence and tumor characteristics with ablative therapies and surgical resection. Table 1. Characteristics of the study groups before and after matching.
Table 1. Characteristics of the study groups before and after matching. Table 2. Multivariate analysis using Cox regression models for overall survival and disease-free survival, adjusting for potential confounders in the study groups before matching.
Table 2. Multivariate analysis using Cox regression models for overall survival and disease-free survival, adjusting for potential confounders in the study groups before matching. Table 3. The pattern of hepatocellular carcinoma recurrence in all patients after ablative therapies and surgical resection.
Table 3. The pattern of hepatocellular carcinoma recurrence in all patients after ablative therapies and surgical resection. Table 4. Pattern of recurrence and tumor characteristics with ablative therapies and surgical resection.
Table 4. Pattern of recurrence and tumor characteristics with ablative therapies and surgical resection. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








