04 October 2022: Original Paper
Incidence of COVID-19 and Identification of Possible Risk Factors Associated with COVID-19 in Acute Renal Transplant Recipients in Pakistan
Nida SaleemDOI: 10.12659/AOT.937688
Ann Transplant 2022; 27:e937688
Abstract
BACKGROUND: Renal transplant recipients are susceptible to increased mortality with COVID-19 infection. There is insufficient data regarding risk factors for COVID-19 disease acquisition. We aimed to identify them here.
MATERIAL AND METHODS: We enrolled Pakistani renal transplant recipients from February 10, 2020, to March 18, 2021, and actively tracked their baseline health status, transplant characteristics, comorbidities, immunosuppressive therapies, and post-transplant follow-ups until September 2021. Furthermore, we formulated 2 questionnaires for their compliance assessment with COVID-19-preventive measures. We also identified COVID-19 disease acquisition, symptomatology, and management.
RESULTS: Among the 50 enrolled patients, 14 (28%) patients developed COVID-19, which is higher than the incidence observed in general Pakistani population (0.55%). Their mean age was 35.38 years ±11.69 SD years, and 82% of patients were males. The following factors were independently associated with COVID-19 disease: female gender (P value: 0.042), diabetes mellitus (P value: 0.002), anti-thymocyte globulin (ATG) induction (P value: 0.006), in-person follow-ups (P value: 0.000), prolonged immediate and late post-transplant hospital stays (P value: 0.019 and 0.000, respectively), raised post-transplant serum creatinine (P value: 0.019), and COVID-19 protective measures non-compliance (P value: 0.000). Out of 14 infected recipients, 92.85% required symptomatic management and overall mortality was 0%.
CONCLUSIONS: Female gender, diabetes mellitus, ATG induction, in-person follow-ups, prolonged hospital stays, raised post-transplant serum creatinine, and COVID-19-protective measures non-compliance were associated with the higher acquisition of SARS-CoV-2 infection. By taking concrete measures against these risk factors, we can continue renal transplants, as overall mortality was lower than in the general Pakistani population (2%).
Keywords: COVID-19, Immunosuppression, Incidence, Kidney Transplantation, Telemedicine, Adult, Antilymphocyte Serum, Creatinine, Diabetes Mellitus, Female, Humans, Male, Pakistan, Risk Factors, SARS-CoV-2, transplant recipients
Background
The novel coronavirus (SARS-CoV-2) pandemic has affected 221 countries around the world. COVID-19 was officially declared a pandemic on March 11, 2020 [1]. More than 3.4 billion people around the world have been affected. The Pakistani population has suffered severely from this pandemic. Since February 2020, 1.3 million Pakistanis have had COVID-19. From February 2020 to September 2021, there were around 1.2 million diagnosed cases. There have been 4 major COVID-19 peaks during this period in Pakistan.
Around the world, this pandemic halted transplant programs due to fear of exposing transplanted, highly immunocompromised patients to this extremely contagious viral infection [2]. Solid organ transplant recipients are exclusively considered a very high-risk population [3] due to chronic immunosuppression, multiple comorbidities, and greater frailty that poses a significant risk of unfavorable outcomes, but renal transplant is considered the best modality of renal replacement therapy [4], with prolonged survival and improvement in quality of life [2].
A study [5] found that COVID-19 affected chronic hemodialysis patients more than renal transplant recipients due to greater exposure. In Pakistan, the incidence of end-stage renal disease is likely to be much higher than reported. Due to poverty and lack of health infrastructure, only 72 hemodialysis units are currently functioning in Pakistan. Even twice-weekly hemodialysis is unaffordable. Besides this, in Pakistan, infections are more common among patients on hemodialysis due to inadequate dialysis, malnutrition, and frequent blood transfusion for persistent anemia [6]. Therefore, in the absence of a home dialysis modality, renal transplantation remains the most suitable option for these patients, as it promotes reduced exposure to COVID-19.
Most published studies have described overall outcomes, including prevalence, symptomatology, risk factors for severity, and mortality [7,8] associated with COVID-19 in a chronic renal transplant population, but there is limited information on incidence, identification of risk factors for COVID-19 [9], and outcome [2] in acute renal transplant recipients. In contrast to new renal transplant recipients, chronic renal transplant recipients have less exposure to this infection due to less frequent hospital follow-ups [5]. Secondly, in comparison to the acute transplant population, the effect of induction immunosuppression has been weaned off in them. Thirdly, recent renal transplant recipients have a possible risk of nosocomial acquisition of COVID-19 [4].
The main aim of this study was to identify new cases of COVID-19 reported in CKD-5 patients who underwent acute renal transplant from February 2020, which was the beginning of the COVID-19 pandemic in Pakistan, until September 2021. Another objective was to identify particular risk factors that could lead to COVID-19 in this population group. We also assessed and scored the preventive measures taken by our acute renal transplant recipients against COVID-19 and whether these measures played a significant role in protecting these recipients from COVID-19.
Material and Methods
RENAL TRANSPLANT PROTOCOL DURING COVID-19 PANDEMIC:
During this pandemic, the following in-hospital COVID-19 preventive measures were taken to reduce transmission of COVID-19 infection.
MAINTENANCE IMMUNOSUPPRESSION:
These renal transplant recipients received a combination regimen of steroids, mycophenolate mofetil (MMF), and tacrolimus. The standard doses of steroids included 500 mg of pulse methylprednisolone for 3 immediate post-transplant days, followed by conversion to oral steroids starting from 60 mg once daily and then gradual tapering to 5 mg once daily throughout 6 months. We maintained tacrolimus trough levels at 6–8 ng/mL and continued MMF at a dose of 2 g per day.
COVID-19 DISEASE:
We diagnosed COVID-19 with a positive nasal or pharyngeal swab real-time quantitative polymerase chain reaction (PCR). Besides this, we inquired about the diagnosis of COVID-19 acquisition, severity, and subsequent management. Afterward, we cross-referenced their answers with our electronic hospital record systems and hospital files.
SEVERITY OF COVID-19 DISEASE:
Based on this study [10], we categorized COVID-19 into 3 groups;
QUALITATIVE SURVEYS:
Two surveys were designed based on the available evidence on COVID-19 and its impact specifically on kidney transplant recipients.
In the first survey, we focused on the appraisal of renal transplant recipients on their awareness of the non-pharmacological interventions for the curtailment of SARS-CoV-2 infection. We asked 2 screening questions to evaluate their knowledge of the current pandemic and vulnerability as recipients of solid organ transplants. Subsequently, we inquired about their awareness of the non-pharmacological measures and scored them. For part A of the questionnaire, we scored each positive answer as 1 and each negative answer as 0. We then calculated the total awareness score by adding responses to all the questions. We considered a score below 10 as a poor score and above 10 as a good score.
In another survey, we aimed to detect patient compliance with non-pharmacological interventions. We divided this survey into 5 sections: level of education, avoidance of exposure to SARS-CoV-2, compliance with protective measures, level of social distancing, and COVID-19 symptomatology. We converted their responses into binary variables (eg, 0, 1, 2, 3, 4, 5). Subsequently, we assigned questions in this part B of the questionnaire an individual score for each response. A low score indicated a higher degree/risk of exposure or non-compliance to preventive measures, and a higher score indicated better compliance. We scored their level of education a maximum score of 2, SARS-CoV-2 exposure avoidance had a maximum score of 15, measures of social distancing had a maximum a score of 11, protective measures had a maximum a score of 9, and inquiry regarding COVID-19 symptomatology had a maximum score of 2, with a highest possible score of 38. Finally, we denoted a score of over 30 as excellent, 20–29 as good, and less than 20 as poor.
QUANTITATIVE DATA:
We also collected data from hospital files of all patients to identify potential risk factors for COVID-19 disease. From these data, we obtained information on patient demographic features, comorbidities, ESRD cause, HLA mismatches, duration of hemodialysis, previous transplants, type of induction immunosuppression, any subsequent post-transplant hospital admission, and number and mode of follow-ups post-transplant.
STATISTICAL ANALYSIS:
We recorded all data and analyzed it using SPSS version 24.0. We presented categorical variables like pre-transplant and post-transplant characteristics as frequencies and percentages. Means and standard deviations were calculated for quantitative variables.
The baseline characteristics, awareness, and implementation score including each variable, of the SARS-CoV-2-positive and -negative groups were compared using the chi-square test (or Fisher’s exact test for smaller counts). Mean serum creatinine, the number of post-transplant follow-ups, length of immediate post-transplant hospital stay, and length of hospital stay during post-transplant admissions of the 2 groups were compared using an independent samples
Results
Table 1 shows the baseline pre- and post-transplant characteristic features of 50 recipients. The mean follow-up period was 12 months ±5 standard deviation (SD). Among these 50 recipients, 41 were males (82%) and 9 were females (18%). Their mean age was 35.38 years ±11.69 SD. Their mean BMI was 23.673 kg/m2 ±4.5685 SD. We found 92% of these recipients were hypertensive, 38% were diabetics, and 24% were smokers. Among the causes of end-stage renal disease (ESRD), 30% had glomerulonephritis, 20% had hypertensive nephropathy, 18% had diabetic nephropathy, and 16% had obstructive nephropathy. Twenty-nine out of 50 patients were on hemodialysis for less than 1 year. Eight patients had undergone pre-emptive renal transplants. Concerning tissue typing, 18% had complete HLA matches and 56% had less than 5 HLA mismatches. None had pre-existing donor-specific antibodies (DSA).
During the immediate post-renal transplant period, 29 out of 50 recipients received 4 doses of ATG, 14 received 2 doses of basiliximab, and the remaining 7 received no induction therapy. Thirty-two out of 50 patients had a short immediate post-transplant hospital stay of fewer than 7 days. Mean post-transplant serum creatinine on discharge was 1.20 mg/dL ±0.41 SD. During the follow-up period, 22% of recipients developed new-onset diabetes after transplant (NODAT). Twenty-four percent of recipients had undergone allograft biopsy. Histopathological evidence of these biopsies showed acute tubular necrosis (ATN) in 8 out of 12 biopsies and allograft rejection in only 3. Eighteen out of 50 patients were admitted post-transplant during the follow-up period. In the first 6 months post-transplant, 74% of recipients followed up more than 10 times. Fifty-eight percent of recipients followed up via telemedicine besides face-to-face follow-up mode.
Table 2 shows SARS-CoV-2 infection outcome and vaccination received by these recipients. As seen in Table 2, the overall incidence of SARS-CoV-2 infection in renal transplant recipients was 28% during a mean follow-up of 12 months. The incidence of COVID-19 during the 6 months follow-up period was 12%. Most recipients were diagnosed with SARS-CoV-2 infection within a mean period of 7.35 months. Among these 14 diagnosed cases, 11 had mild symptoms (78.6%), 3 had moderate symptoms (21.42%), and none had severe symptoms. Among 14 infected recipients, 13 required conservative management (92.86%). Only 1 recipient was admitted to the hospital and received anti-viral therapy. None needed invasive and non-invasive ventilatory support. No recipient died due to COVID-19. However, 1 recipient died due to cardiac arrest 7 months post-transplant.
Regarding COVID-19 vaccination (Table 2), 31 out of 50 recipients were vaccinated in the mean period of 9.77 months. Only 3 out of these 31 recipients got vaccinated before SARS-CoV-2 infection. One got vaccinated immediately before infection acquisition. The remaining 2 received vaccination 1 and 2 months before infection. We found that 54.8% of recipients received inactivated vaccine, 38.7% received RNA, and 6.45% received viral vector COVID-19 vaccination. None out of these 31 vaccinated recipients reported any complications post-vaccination.
Table 3 shows a detailed interpretation of possible risk factors for SARS-CoV-2 infection acquisition in renal transplant recipients. Female gender was positively associated with COVID-19 infection acquisition (
Table 4 shows other risk factors for acquiring SARS-CoV-2 infection post-transplant. As seen, mean lengths of immediate and delayed post-transplant hospital stay were significantly associated with the acquisition of SARS-CoV-2 infection (
Concerning non-pharmacological interventions, the occurrence of SARS-CoV-2 infection has been linked to both awareness and implementation scores, as shown in Table 5.
As shown above, only 3 out of 50 patients had an awareness score of less than 10. There was no association of awareness score with SARS-CoV-2 infection (
Discussion
This retrospective study provides a comprehensive insight into COVID-19 disease in acute consecutively selected renal transplant recipients. With a detailed description of 50 new kidney transplant recipients, we can estimate the incidence of COVID-19 disease, risk factors for its acquisition, its symptomatology, and a brief description of its management over the mean follow-up period of 12 +5 months, which corresponded to the 4 peak phases of the epidemic in Pakistan.
According to Worldometer statistics [11], from February 2020 to September 2021, 1.2 million COVID-19 cases have been reported in Pakistan, out of 220 million total Pakistani population. The calculated incidence of COVID-19 cases was 0.55%. During this period, the mortality rate due to COVID-19 was 2%. In our acute renal transplant recipients, the calculated incidence was 28%, and the mortality rate was 0% over the mean follow-up period of 12 +5 months. In our acute transplant recipients, although the reported incidence was higher than in the general Pakistani population, the calculated mortality rate was lower than in the general Pakistani population.
Few studies have described several risk factors associated with increased susceptibility to COVID-19. These include diabetes mellitus, obesity, chronic pulmonary disease, hypertensive kidney disease, deep venous thrombosis, Asian ethnicity, AB blood group, and high trough levels [12,13]. This study has described certain potential modifiable and non-modifiable risk factors for COVID-19 disease acquisition. Our study indicates the significant role of the 2 non-modifiable factors in the SARS-CoV-2-related disease in acute kidney transplant recipients: diabetes mellitus and female gender. Previous studies have also described the association of diabetes mellitus with COVID-19 acquisition [5,13]. However, many of these previous studies have described the risk of COVID-19 in long-term renal transplant recipients. From this study, we can conclude that diabetes mellitus is associated with an increased risk of COVID-19 in both short and long-term renal transplant recipients. Therefore, we suggest that every diabetic pre-transplant recipient should be considered a high-risk patient. It must be mandatory to counsel them regarding the strict observance of COVID-19 preventive and protective measures.
Several studies have described the association of raised serum creatinine with mortality secondary to COVID-19 [10]. In this study, we postulate that raised serum creatinine or delayed graft function during the immediate post-transplant period is linked to an increased risk of SARS-CoV-2 infection, probably due to prolonged post-transplant hospital stay and subsequently greater exposure to SARS-CoV-2. Another study supports this proposition [5]. However, this needs further validation by other observational studies.
In multiple studies [14,15], the potential role of telemedicine follow-ups has been encouraged and considered a reasonable follow-up approach. Telehealth follow-ups have several advantages, including feasibility, convenience, less time, and lower cost [16]. Our study has proved these recommendations, as recipients with face-to-face follow-ups have developed COVID-19. Therefore, it can be considered a mandatory step to reduce the transmission of SARS-CoV-2 among severely immunocompromised renal transplant recipients.
Regarding ATG, based on its lymphocytic depleting property, published data suggested its association with the increased risk of viral infections like SARS-CoV-2 [17]. A previously published study [18] using ATG Sanofi suggested using ATG on an as-needed basis during this pandemic. As this study shows a clear association between the use of ATG and SARS-CoV-2 infection acquisition, we conclude that the use of ATG at a cumulative, low dose of 6 mg/kg instead of 9 mg/kg, should only be prescribed in recipients with high immunological risk. Use of ATG-F must be encouraged rather than rabbit ATG (r-ATG) and thymoglobulin (ATG Sanofi) [19] due to its low lymphocytic suppressive ability. In recipients with low immunological risk, we encourage the substitution of basiliximab for ATG as a non-lymphocytic depleting agent. During this pandemic, avoidance of induction immunosuppression, as seen in our 7 recipients, can be tried in case of a complete allograft match.
Several published studies scored awareness about COVID-19 among the transplant population [3,9]. In our study, 47 out of 50 recipients were highly aware of the ongoing COVID-19 pandemic. However, only 10 recipients had excellent implementation scores. These findings indicate that an ongoing mandatory implementation of COVID-19 preventive measures is necessary for the recipients to protect themselves from this life-threatening infection. Therefore, the transplant coordinator and the managing physician must ensure compliance with COVID-19 preventive measures by frequently reminding recipients about them. In addition, following protective measures like covering the face with masks, using hand sanitizers, and frequent hand washing is significantly associated with reduced incidence of COVID-19. Therefore, we suggest implementing them relentlessly.
Based on the efficacy of COVID-19 vaccination in halting severe COVID-19 in the general population, many studies supported their use. However, they also raised several concerns like the potential for reduced efficacy in immunocompromised recipients, uncertainty regarding the durability of the immune response, and potential risk for allograft rejection after vaccination [20]. Sixty-two percent of our recipients got vaccinated at around 9 months post-transplant. Furthermore, there was no post-vaccination complication and allograft rejection. In one study [21], 15 out of 55 (27%) transplant recipients developed severe COVID-19 even after receiving 2 doses of the mRNA vaccine. A potential explanation for increased disease susceptibility despite vaccination is an absence of adequate humoral response due to ongoing immunosuppression. Therefore, we suggest that, as we observed no serious complications of COVID-19 vaccinations in our recipients, a trial of increased or double dose of vaccination can be given. However, this proposition needs further validation.
In studies conducted in France [13] and Qatar [12], the incidence and mortality associated with COVID-19 in renal transplant recipients were higher than in the general population. Among our transplant recipients, the incidence was higher than in the general Pakistani population. However, there was reduced mortality in our COVID-19 patients. Furthermore, a study suggested that there is variability in clinical predisposition, presentation, and outcome of COVID-19 infection among different renal transplant populations [22]. However, these findings cannot be considered conclusive due to the small sample size of our SARS-CoV-2-infected transplant recipients.
By evaluating the risk factors for COVID-19 and identifying COVID-19 outcomes in acute transplant recipients over a mean follow-up of approximately 1 year, we found certain salient features that require further investigation. Firstly, we found a potential role of ATG in COVID-19 acquisition and the need to formulate guidelines regarding its limited use, especially in patients with complete allograft matches. Secondly, it is mandatory to enhance telemedicine follow-ups in non-urgent cases, and recipients must be thoroughly counseled and directed regarding these follow-ups. Thirdly, as diabetic females are at high risk for COVID-19 acquisition, it is necessary to instruct them regarding adequate diabetic control and compliance with protective measures. Lastly, transplant physicians and coordinators should consider it their responsibility to remind recipients about uninterrupted compliance with COVID-19 preventive strategies.
The main limitations of this study are the small sample size and the retrospective nature of the study. Findings reported in this study, especially related to risk factors for COVID-19 occurrences in acute renal transplant recipients, should be further investigated and validated in a large sample of newly transplanted recipients.
Conclusions
Although, as noted, the incidence of COVID-19 in renal transplant recipients is higher than in the general Pakistani population, the overall mortality associated with COVID-19 in acute renal transplant recipients has been low. Therefore, we suggest that it is prudent to continue performing renal transplants by strictly following the use of personal protective measures, by risk categorization of recipients, minimizing or avoiding ATG after rejection risk assessment, trying to minimize hospital admissions, and avoiding prolonged immediate and late in-hospital stays, and lastly, by promoting teleconsultations, especially in non-urgent cases.
Tables
Table 1. Pre and post-transplant characteristics of kidney transplant recipients.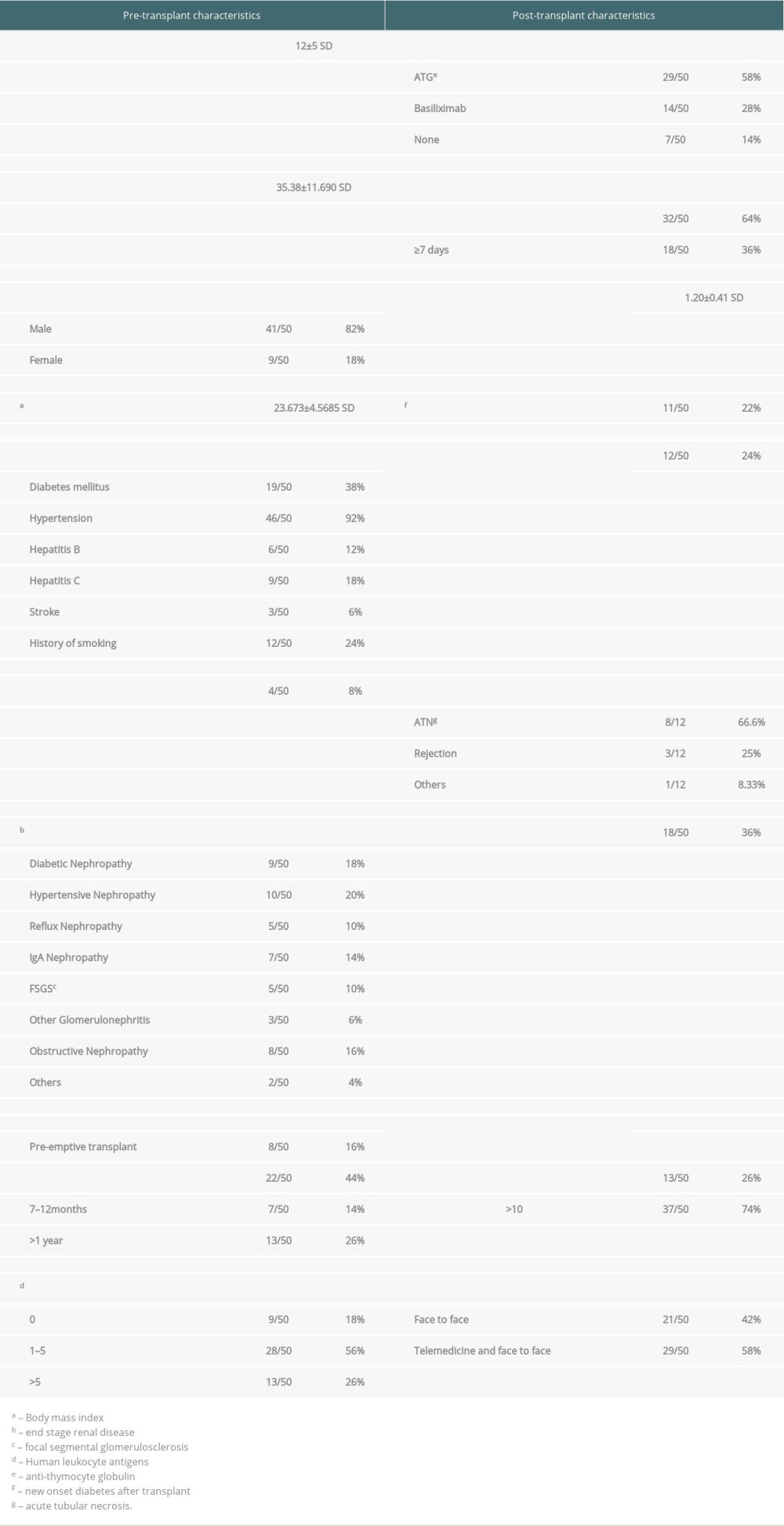 Table 2. COVID-19 infection and vaccination data.
Table 2. COVID-19 infection and vaccination data.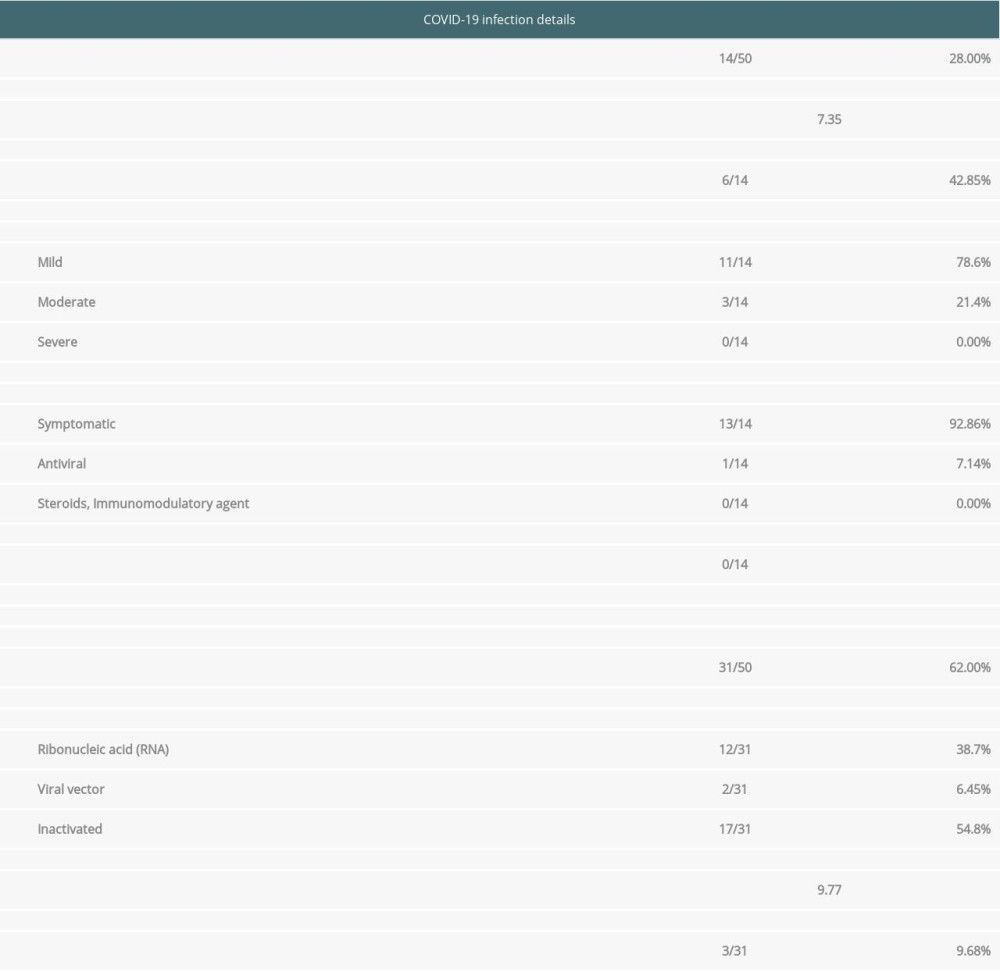 Table 3. Baseline transplant characteristics by COVID-19 status.
Table 3. Baseline transplant characteristics by COVID-19 status.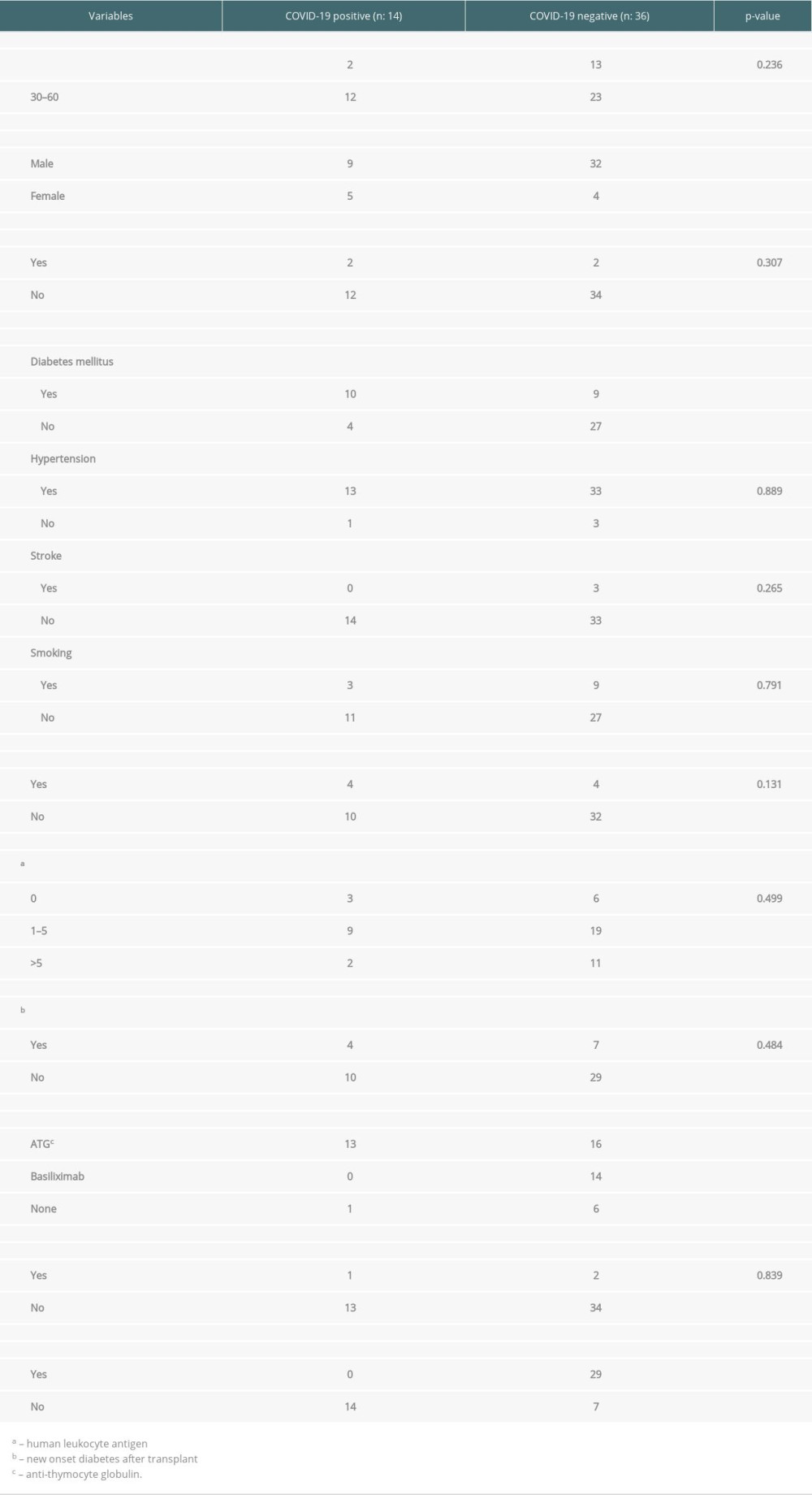 Table 4. Linking Quantitative variables with COVID-19 status.
Table 4. Linking Quantitative variables with COVID-19 status.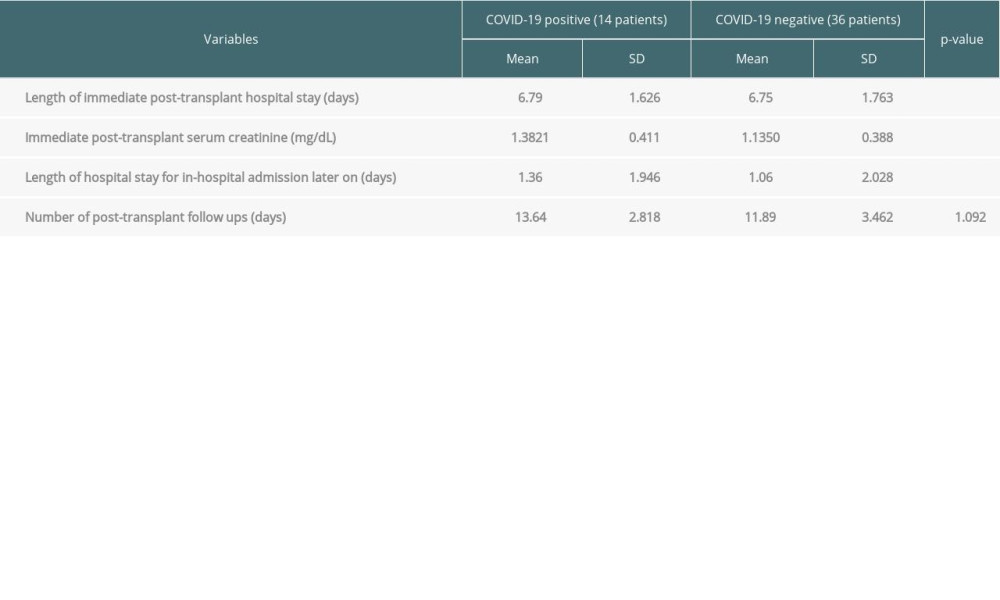 Table 5. Linking Qualitative surveys results with COVID-19.
Table 5. Linking Qualitative surveys results with COVID-19.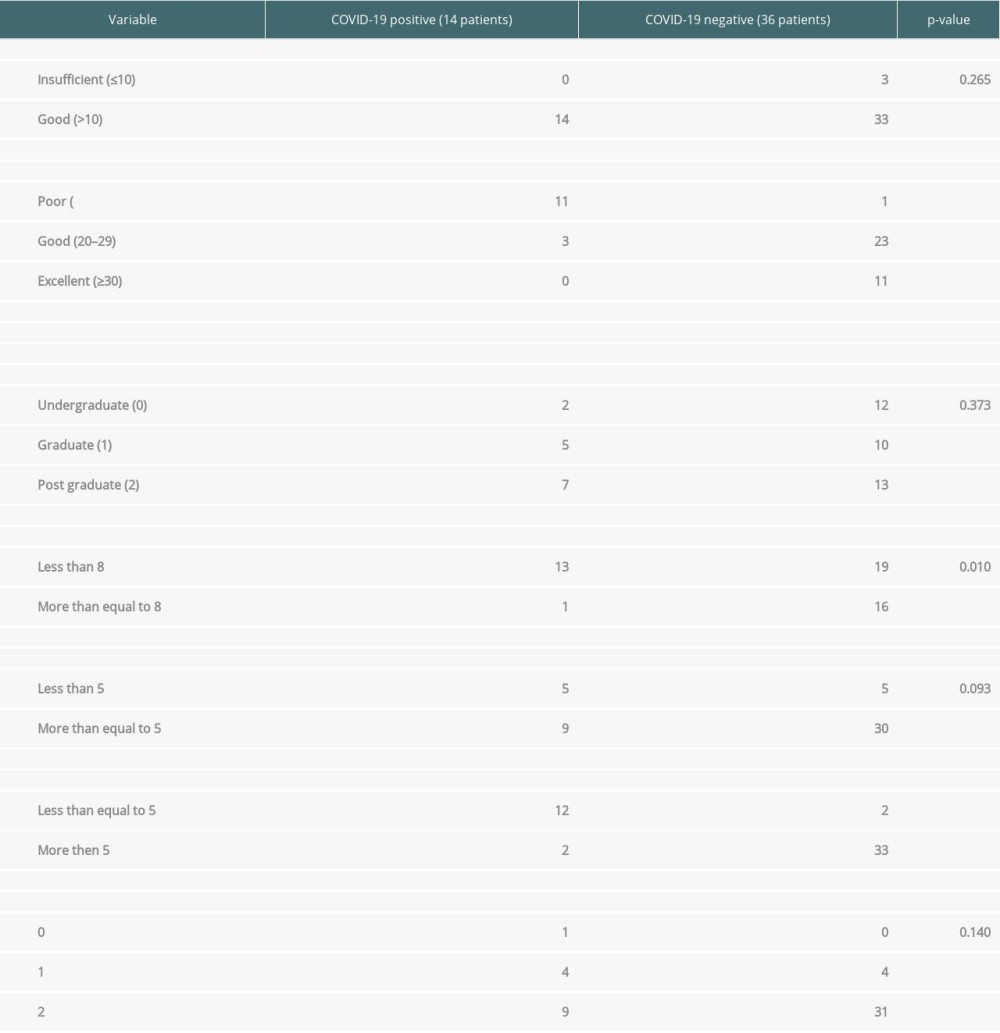
References
1. Chung SJ, Tan EK, Kee T, Practical considerations for solid organ transplantation during the COVID-19 global outbreak: The experience from Singapore: Transplant Direct, 2020; 6(6); e554
2. Laessle C, Schneider J, Pisarski P, Experiences and short-term outcomes of kidney transplantation during the coronavirus disease 2019 pandemic from a medium-volume transplantation and Superregional Coronavirus Disease 2019 Treatment Center: Transplant Proc, 2021; 53(4); 1146-53
3. Hasanoglu I, Bilgic Z, Olcucuoglu E, Do lifestyle changes of renal transplant recipients during the pandemic reduce the risk of coronavirus disease 2019?: Transplantation Proceedings, 2020; 52(9); 2667-70
4. Clarke C, Lucisano G, Prendecki M, Informing the risk of kidney transplantation versus remaining on the waitlist in the coronavirus disease 2019 era: Kidney Int Rep, 2021; 6(1); 46-55
5. Clarke C, Lucisano G, Prendecki M, Informing the risk of kidney transplantation versus remaining on the waitlist in the coronavirus disease 2019 era: Kidney Int Rep, 2021; 6(1); 46-55
6. Sakhuja V, Sud K, End-stage renal disease in India and Pakistan: Burden of disease and management issues: Kidney Int Suppl, 2003(83); S115-18
7. Ge E, Li Y, Wu S, Candido E, Wei X, Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study: PLoS One, 2021; 16(10); e0258154
8. Zaki N, Alashwal H, Ibrahim S, Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: A systematic review: Diabetes Metab Syndr, 2020; 14(5); 1133-42
9. Monaco A, Manzia TM, Angelico R, Awareness and impact of non-pharmaceutical interventions during coronavirus disease 2019 pandemic in renal transplant recipients: Transplant Proc, 2020; 52(9); 2607-13
10. Bhandari G, Tiwari V, Gupta A, COVID-19 infection in renal transplant patients: Early report From India: Indian J Nephrol, 2021; 31(3); 271-75
11. Alimetov A: Worldometer [Real-time statistics], United States of America [Available from: https://www.worldometers.info/coronavirus/country/pakistan/
12. Alkadi MM, Al-Malki HA, Asim M, Kidney transplant recipients infected with coronavirus disease 2019: Retrospective Qatar experience: Transplant Proc, 2021; 53(8); 2438-46
13. Elias M, Pievani D, Randoux C, COVID-19 infection in kidney transplant recipients: Disease incidence and clinical outcomes: J Am Soc Nephrol, 2020; 31(10); 2413-23
14. Alshaqaq A, Al Abadi A, Altheaby A, Coronavirus disease 2019 and kidney transplantation in Saudi Arabia: Outcomes and future opportunities: Ann Transplant, 2021; 26; e931832
15. Bach-Pascual A, Pedreira-Robles G, Perez-Saez MJMonitoring and telematic control of people with kidney transplant and suspected COVID-19 infection: Rev Esp Salud Publica, 2021; 95; e20213043 [in Spanish]
16. Huuskes BM, Scholes-Robertson N, Guha C, Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic: Transpl Int, 2021; 34(8); 1517-29
17. George J, Gracious N, Gopal A, Low-dose induction immunosuppression in deceased donor kidney transplantation during coronavirus disease pandemic – a multicentric prospective observational study: Ind J Trans, 2021; 15(4); 313
18. Kolonko A, Więcek A, Safety of antithymocyte globulin use in kidney graft recipients during the COVID-19 pandemic: Ann Transplant, 2021; 26; e933001
19. Song T, Yin S, Li X, Thymoglobulin vs. ATG-fresenius as induction therapy in kidney transplantation: A Bayesian network meta-analysis of randomized controlled trials: Front Immunol, 2020; 11; 457
20. Aslam S, Goldstein DR, Vos R, COVID-19 vaccination in our transplant recipients: The time is now: J Heart Lung Transplant, 2021; 40(3); 169-71
21. Caillard S, Chavarot N, Bertrand DKFrench Society of Transplantation, Occurrence of severe COVID-19 in vaccinated transplant patients: Kidney Int, 2021; 100(2); 477-79
22. Fishman JA, The immunocompromised transplant recipient and SARS-CoV-2 infection: J Am Soc Nephrol, 2020; 31(6); 1147-49
Tables
 Table 1. Pre and post-transplant characteristics of kidney transplant recipients.
Table 1. Pre and post-transplant characteristics of kidney transplant recipients. Table 2. COVID-19 infection and vaccination data.
Table 2. COVID-19 infection and vaccination data. Table 3. Baseline transplant characteristics by COVID-19 status.
Table 3. Baseline transplant characteristics by COVID-19 status. Table 4. Linking Quantitative variables with COVID-19 status.
Table 4. Linking Quantitative variables with COVID-19 status. Table 5. Linking Qualitative surveys results with COVID-19.
Table 5. Linking Qualitative surveys results with COVID-19. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








