09 January 2024: Original Paper
Retrospective Analysis of the Impact of High- and Low-Quality Donor Livers for Patients with High-Acuity Illness
Ron K. VargheseDOI: 10.12659/AOT.941931
Ann Transplant 2024; 29:e941931
Abstract
BACKGROUND: Patients with high-acuity liver failure have increased access to marginal and split liver options, owing to historically high waitlist mortality rates. While most research states that donor liver quality has no impact on patients with high-acuity illness, there have been inconsistencies in recent research on how liver quality impacts post-transplant outcomes for these patients. We aimed to quantify donor liver quality with various post-transplantation patient outcomes for patients with high-acuity illness.
MATERIAL AND METHODS: Using the liver donor risk index (LDRI), model for end stage liver disease (MELD), and clinically relevant recipient factors, we used multivariate logistic regression to analyze how donor liver quality affects varying measures of patient outcomes for 9923 high-acuity patients from June 18, 2013, to June 18, 2022.
RESULTS: Using LDRI, high-quality livers had a significant protective impact on high-acuity patient mortality, compared with low-quality livers (OR=0.695 [0.549, 0.879], P=0.002). High-quality livers also had significant impact on graft survival (OR=0.706 [0.558, 0.894], P=0.004). Two sensitivity patient mortality analyses, excluding patients with status 1A and hepatocellular carcinoma, showed significant protective findings for high-quality livers. High-quality livers had insignificant outcomes on long-term survivor mortality, length of hospitalization, and primary non-function outcomes, compared with low-quality donor livers.
CONCLUSIONS: While our findings suggest donor quality has an impact on high-acuity patient outcomes, these findings indicate further research is needed in intent-to-treat analysis on clinical offer data to provide a clearer finding of how donor quality affects patients with high-acuity illness.
Keywords: Donor Selection, Patient Acuity
Background
Current donor liver shortage has led to an increase in the severity of illness, liver disease, and waiting times for patients. To prioritize and rank patients with high-acuity illness, the model for end stage liver disease (MELD) is a score index created to highlight high-acuity illness through adverse waitlist outcomes [1]. Due to the high risk of waitlist mortality for patients with high-acuity illness, aggressive utilization efforts have been taken to increase the access of marginal and split liver donors [2–4]. In an attempt to quantify donor liver quality, the liver donor risk index (LDRI), a metric measuring the quality of donor livers, is used; studies have commonly used and validated this index to quantify liver quality for outcomes analysis [5–7]. The LDRI is an effective predictive tool, compared with past indexes such as the extended donor criteria, with several studies affirming the validity of LDRI in determining donor quality against post-transplant outcomes [3–5]. Research has analyzed the effect of donor liver quality on the outcomes of patients with high-acuity illness, which mainly remain inconclusive regarding the impact of organ quality on post-transplant outcomes. For example, many studies indicate there is no survival benefit to using either high- or low-quality organs, arguing to take the first available liver [6–12]. Research has indicated that post-transplant graft survival outcomes are independent of donor quality for patients with high MELD scores [7,13]. In contrast, some studies suggest that patients with high-acuity illness receive substantial post-transplant survival benefits with high-quality livers, with low-quality grafts presenting danger to short-term graft survival due to risk factors such as primary non-function (PNF), which is irreversible graft failure within 14 days [14,15]. Studies have found that the use of marginal donor organs in patients with high-acuity illness has led to early patient mortality and graft failure, suggesting that combining impaired donor grafts and recipients should be avoided [16,17].
The aim of this study was to quantify the post-transplant impact of transplanting high- or low-quality organs in patients with high-acuity illness. We believe that by using MELD, LDRI, and clinically relevant recipient factors this study can help clarify the impact of donor quality on post-transplant outcomes for patients with high-acuity illness, as well as to amend the paucity of information of previous research.
Material and Methods
STUDY POPULATION:
We performed a retrospective analysis of data provided by the United Network for Organ Sharing database for patients receiving liver transplants from June 18, 2013, to June 18, 2022. We excluded patients aged under 18 years from the study population. We also excluded patients with a living donor transplant, patients undergoing multiple organ transplant, patients with a MELD score under 35, patients undergoing retransplant, patients with history of non-liver transplant, and patients with a familial relation to the donor from the study (Figure 1). Patients were followed from the date of transplant through death or loss to follow-up. The main study population consisted of 9923 liver transplant patients with high-acuity illness across 9 years (Table 1).
ETHICS APPROVAL:
Patient consent and study approval were waived by the Institutional Review Board of Baylor College of Medicine because patient information was de-identified and not reported in the study, to preserve patient confidentiality.
STATISTICAL ANALYSIS:
Data analyses were performed using the standard statistic software, Stata 17.0 (Stata Corp, College Station, TX, USA). All continuous variables are shown as mean±standard deviation. LDRI was calculated from the study population, and the patients were stratified based on their LDRI scores. High-quality donor liver recipients were classified as the top 20% of LDRI scores (LDRI<1.33), and low-quality donor liver recipients were classified as the bottom 20% of LDRI scores (LDRI>1.86). Recipients of normal-quality donor livers made up the middle 60% (1.33<LDRI<1.86).
ANOVA tests were performed across the 3 groups to measure differences among continuous variables, while chi-square tests were performed to measure differences among categorical variables. Univariate and multivariate logistic regression were performed. We observed the following 7 primary outcomes in separate analyses: patient mortality, graft survival, length of hospitalization, long-term (1-year) survivor patient mortality, non-status-1A mortality, non-hepatocellular carcinoma (HCC) mortality, and PNF. Patient mortality, graft survival, non-status-1A mortality, and non-HCC mortality were evaluated for 1-year mortality. Length of hospitalization within 30 days was assessed. Long-term survivor patient mortality was evaluated past 1 year of survival. PNF within 14 days after transplantation was evaluated. Post-transplant mortality, graft survival, and long-term survivor patient mortality outcomes were evaluated with 8-year Kaplan-Meier curves and log-rank test methods. Patients lacking data on discharge date were omitted from length of hospitalization analysis (n=214).
Low-quality livers were defined as the reference group, with high- and normal-quality livers being compared against them. This was done to show the difference between choosing higher quality livers against low-quality livers. High- and normal-quality livers were included in all multivariate logistic regression analyses, even if not found to be significant in univariate analyses. This was done since this paper focuses on the impact of allograft quality. For all other donor and recipient variables evaluated (Table 2), univariate covariates were included only in multivariate analyses if they had a univariate P value less than 0.05. The tables show the results of these multivariate analyses. Primary outcomes were represented as odds ratios (ORs) with 95% confidence intervals. An OR<1 indicated that there were decreased odds of an event happening. For example, an OR<1 for post-transplant mortality within 1 year meant there were decreased odds of mortality within 1 year for the group in testing (high- or normal-quality donor liver recipients) against the reference (low-quality donor liver recipients). Conversely, OR>1 indicated increased odds of mortality within 1 year for groups in testing against the reference.
To account for the potential bias of patients with a worse prognosis receiving low-quality allografts, status 1A patients were excluded (n=746), and the population study was evaluated in a separate multivariate logistic regression analysis for post-transplant mortality within 1 year. Patients with status 1A have acute liver failure, require a transplant within 7 days, and are given the highest medical priority to receive donor livers [18]. This sensitivity analysis provided better interpretation of the odds ratios for low- and high-quality livers (LDRI<1.33 and LDRI>1.86) in short- and long-term patient mortality. Multivariate logistic regression was done with variables displaying significant univariate odds ratios as aforementioned.
Research has shown patients with liver failure involving liver malignant tumors are done in a separate analysis [19–22]. Addressing these patients, we performed an additional sensitivity analysis excluding patients with HCC (n=235) from the sample, which left a sample size of 9688 patients with high-acuity illness. This was done since HCC is malignant in nature relative to other causes of liver failure. Univariate and multivariate logistic regression was performed with variables used only in multivariate analysis if found significant under univariate regression.
Results
STUDY POPULATION:
Our study population consisted of 9923 liver transplant patients with high-acuity illness from 2013 to 2022. Table 1 summarizes and stratifies the demographics of these data based on low- (LDRI>1.86), normal- (1.33<LDRI<1.86), and high- (LDRI<1.33) quality donor organs. We found women to be statistically significantly more likely to receive low-quality donor liver allografts and individuals of African American race to be more likely to receive low- and normal-quality donor liver allografts. Furthermore, we found individuals with a higher international normalized ratio to be significantly more likely to receive a low-quality liver allograft. Regarding height and weight, we observed a similar trend of increasing mean values for these variables as the quality of the liver increased. Causes of liver failure were similar between low- and high-quality liver donor recipients. However, alcoholic cirrhosis, hepatitis C, and acute hepatic necrosis occurred more often in normal-quality liver donor recipients than in low- or high-quality donor recipients (Table 1).
PATIENT MORTALITY:
Univariate logistic regression was used to identify risk factors that substantially affected patient mortality (Table 2). Across 9923 patients, high-quality donor livers were more protective than low-quality livers (OR=0.791 [0.654, 0.958], P=0.02; Table 3). Normal-quality donor livers did not significantly affect patient mortality (P=0.78; Table 3). In multivariate analysis, high-quality donor livers showed approximately 30.5% less odds in adjusted 1-year patient mortality than low-quality grafts (OR=0.695 [0.549, 0.879], P=0.02; Table 3). Normal-quality livers did not significantly affect mortality (P=0.10; Table 3). Analysis with 8-year Kaplan-Meier curves and log-rank tests showed significant differences for high- and normal-quality livers, compared with the low-quality reference range for patient mortality (P<0.001 and P=0.02, respectively; Figure 2A). The difference appeared as early as 90 days after transplantation.
GRAFT SURVIVAL:
Univariate testing of graft survival in 9923 patients with high-acuity illness showed high-quality donor livers had significant protective impact, compared with low-quality livers (OR=0.790 [0.653, 0.956], P=0.02; Table 3). Normal-quality donor livers did not significantly affect graft mortality (P=0.82; Table 3). In multivariate analysis, high-quality donor livers had approximately 29.4% less odds in adjusted 1-year graft survival than low-quality grafts (OR=0.706 [0.558, 0.894], P=0.004; Table 3). Normal-quality livers did not significantly affect 1-year mortality (P=0.09; Table 3). Log-rank testing showed significant differences for high- and normal-quality livers, compared with the low-quality reference range for graft mortality (P<0.001 and P=0.02, respectively; Figure 2B).
LENGTH OF HOSPITALIZATION:
Postoperative hospital time is a commonly accepted indicator for morbidity [22]. We therefore evaluated length of hospital stay in relation to graft quality. Neither high- nor normal-quality livers in univariate logistic regression were found to significantly affect postoperative hospitalization time (P=0.72 and P=0.08, respectively; Table 3). The true impact of graft quality on the length of postoperative hospital stay was evaluated by accounting for other clinically relevant factors through multivariate logistic regression. Normal-quality livers showed a significant effect on length of hospitalization (OR=0.845 [0.741, 0.965], P=0.01; Table 3). High-quality liver had no significant effect (P=0.12; Table 3).
LONG-TERM SURVIVOR PATIENT MORTALITY:
To isolate the long-term effects of graft quality on patient survival, long-term post-transplant mortality was evaluated in 9061 patients with high-acuity illness who met the criteria of surviving more than 1 year after transplantation. Univariate logistic regression found that high- and normal-quality livers did not significantly affect long-term survivor patient mortality, compared with low-quality livers (P=0.99 and P=0.98, respectively; Table 3). Multivariate analysis also showed that both high- and normal-quality livers did not significantly affect long-term survivor patient mortality, compared with low-quality livers. (P=0.86 and P=0.81, respectively; Table 3). Log-rank testing also showed insignificance for high- and normal-quality livers against low-quality livers (P=0.19 and P=0.11, respectively; Figure 3).
STATUS 1A SENSITIVITY ANALYSES:
In an attempt to address the potential bias of patients with a worse prognosis receiving a higher number of low-quality allografts, 9177 patients were included in the sensitivity analysis, excluding status 1A patients. In univariate testing, high-quality liver allografts were found to be protective (OR=0.805 [0.659, 0.984], P=0.03; Table 3), compared with low-quality livers. Normal-quality livers had no significant impact (P=0.88; Table 3). Multivariate testing demonstrated 29% decreased odds of 1-year mortality for high-quality compared with low-quality liver allografts (OR=0.703 [0.548, 0.900], P=0.005; Table 3). Normal-quality livers did not have a significant impact (P=0.10; Table 3).
HCC SENSITIVITY ANALYSES:
Of 9923 patients with high-acuity illness, 248 had liver failure associated with HCC. To address bias for prognoses of liver failure malignant in nature, 9675 patients were included in this sensitivity analysis, excluding patients with HCC. There was a statistically significant difference between high-quality and low-quality liver allografts in univariate testing (OR=0.764 [0.603, 0.970], P=0.002; Table 3). Normal-quality livers showed no significant protective impact (P=0.68; Table 3). In multivariate regression, high-quality donor livers showed a significant protective impact (0.736 [0.578, 0.927], P=0.013), compared with low-quality allografts. Normal-quality allografts did not show a significant protective effect (P=0.131; Table 3).
PNF ANALYSES:
Within the population study group of 9923 patients with high-acuity illness, there were 218 patients who had PNF after transplantation. Of these 218 patients, 41, 127, and 50 patients received their livers from high-, normal-, and low-quality donors, respectively. Results of univariate logistic regression were not significant for the selection of high- or normal-quality livers, compared with low-quality allografts (P=0.66 and P=0.59, respectively; Table 3). Multivariate analysis also did not show significance for high- and normal-quality donor livers and PNF (P=0.46 and P=0.35, respectively; Table 3). PNF outcomes occurred proportionally among each type of donor liver quality (Figure 4).
Discussion
LIMITATIONS:
Because the study population originated from a large database through the Organ Procurement and Transplantation Network, the impact of incorrect or missing data should be minimal. This analysis cannot account for the limited options or time constraints placed on patients with high-acuity illness, as further research involving intent-to-treat analysis could better account for this. The variables of the LDRI, while widely used in post-transplant outcome analysis, are created from data during 2006 [28]. A revised LDRI with variables based on recent data could provide better observatory value in donor quality than current high-acuity patient outcomes. Other studies have shown that a significant amount of predictive ability in the LDRI comes from individual variables such as donor age and donor after cardiac death [29]. Additional analysis on such significant drivers of the LDRI could add a more concrete standard of assessing donor quality than LDRI alone on post-transplant outcomes. Patients with PNF were limited. Further stratification of organ quality by the LDRI, instead of the top and bottom 20% of donor livers as presented in the present study, may provide clearer results on how donor liver quality affects patient outcomes.
Conclusions
This comprehensive multivariate analysis of patients with high-acuity illness liver transplantation shows a significant protective impact in choosing high-quality over low-quality donor livers for patient mortality and graft survival after transplantation. Allograft quality did not significantly impact PNF, length of hospitalization, and long-term survival. These findings are of limited scope as they analyze only the post-transplantation impact of allograft quality against patient outcomes; however, this gives merit for further research on the impact of allograft quality for patients with high-acuity illness.
Figures
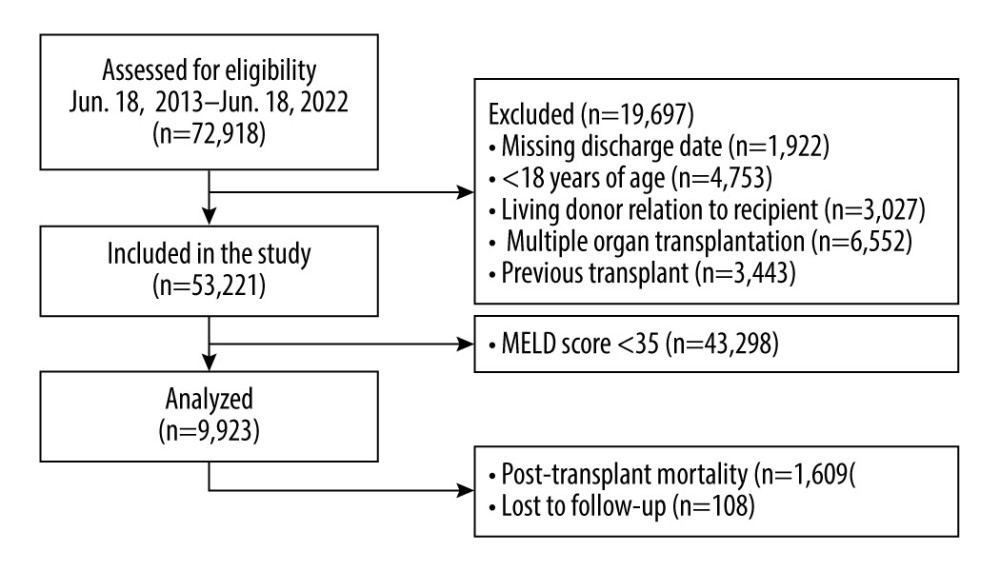 Figure 1. Study population exclusion criteria. A total of 72,918 patients who received liver transplants from June 18, 2013, to June 18, 2022 were assessed for eligibility. The figure displays exclusion criteria used to select the 9923 patients included in this analysis. StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: Stata Corp LLC.
Figure 1. Study population exclusion criteria. A total of 72,918 patients who received liver transplants from June 18, 2013, to June 18, 2022 were assessed for eligibility. The figure displays exclusion criteria used to select the 9923 patients included in this analysis. StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: Stata Corp LLC. 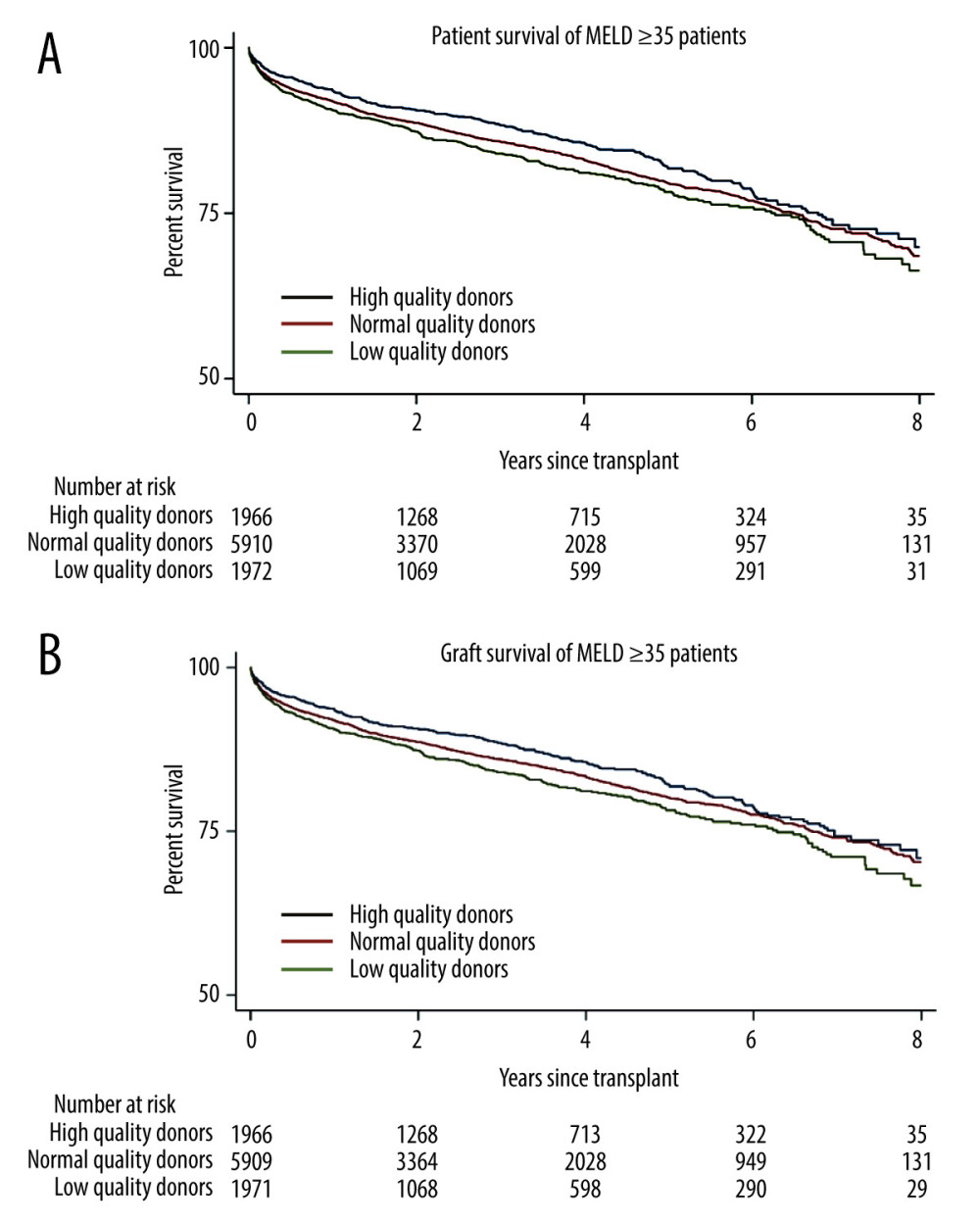 Figure 2. Kaplan-Meier curve for survival in patients with MELD scores ≥35. (A) Patient survival shows significant difference for high- and normal-quality donors, compared with low-quality donors (log-rank, P<0.001 and P=0.02, respectively. (B) Graft survival shows significant differences for high- and normal-quality donors, compared with low-quality donors (log-rank, P<0.001 and P<0.001, respectively). StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.
Figure 2. Kaplan-Meier curve for survival in patients with MELD scores ≥35. (A) Patient survival shows significant difference for high- and normal-quality donors, compared with low-quality donors (log-rank, P<0.001 and P=0.02, respectively. (B) Graft survival shows significant differences for high- and normal-quality donors, compared with low-quality donors (log-rank, P<0.001 and P<0.001, respectively). StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC. 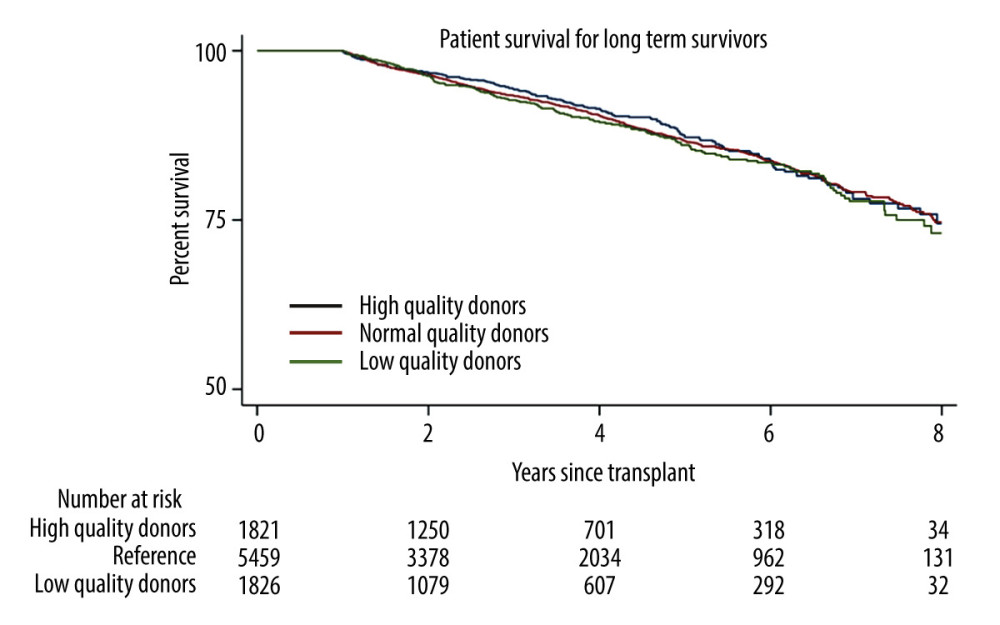 Figure 3. Long-term (1 year) survivor patient mortality. Insignificant differences were found for high- and normal-quality donors, compared with low-quality donors (log-rank, P=0.19 and P=0.11, respectively). StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.
Figure 3. Long-term (1 year) survivor patient mortality. Insignificant differences were found for high- and normal-quality donors, compared with low-quality donors (log-rank, P=0.19 and P=0.11, respectively). StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC. 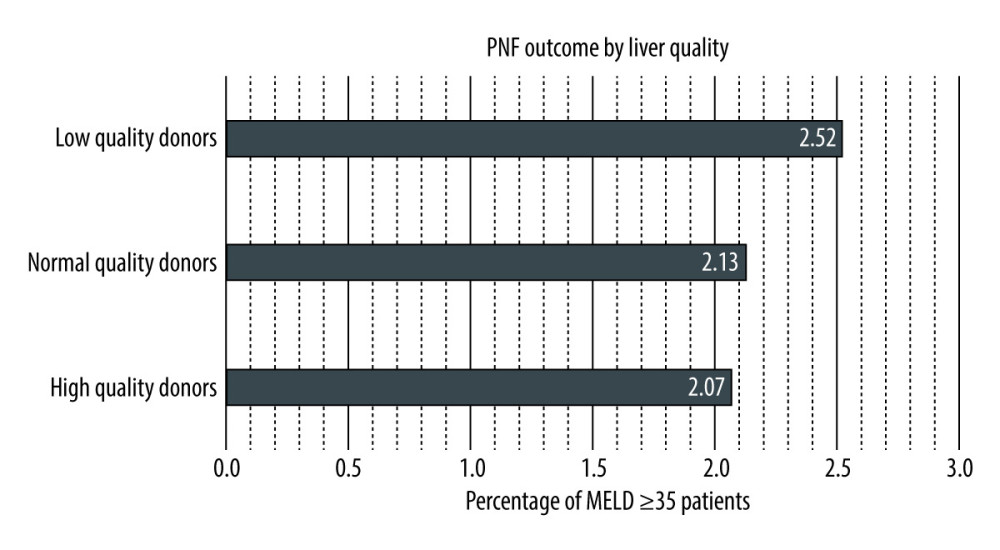 Figure 4. Primary non-function by liver quality. Low-, normal-, and high-quality donors make up 2.52%, 2.13%, and 2.07% of patients, respectively, with MELD scores ≥35. Log-rank testing shows insignificant differences for high- and low-quality donors, compared with normal-quality donors. StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.
Figure 4. Primary non-function by liver quality. Low-, normal-, and high-quality donors make up 2.52%, 2.13%, and 2.07% of patients, respectively, with MELD scores ≥35. Log-rank testing shows insignificant differences for high- and low-quality donors, compared with normal-quality donors. StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC. References
1. Sharma P, Schaubel DE, Gong Q, End-stage liver disease candidates at the highest model for end-stage liver disease scores have higher wait-list mortality than status-1A candidates: Hepatology, 2012; 55(1); 192-98 Erratum in: Hepatology. 2012;55(4):1311
2. Feng S, Goodrich NP, Bragg-Gresham JL, Characteristics associated with liver graft failure: the concept of a donor risk index: Am J Transplant, 2006; 6(4); 783-90 Erratum in: Am J Transplant. 2018;18(12):3085
3. Zhang T, Dunson J, Kanwal F, Trends in outcomes for marginal allografts in liver transplant: JAMA Surg, 2020; 155(10); 926-32 Erratum in: JAMA Surg. 2020;155(10):1002
4. Rana A, Gruessner A, Agopian VG, Survival benefit of solid-organ transplant in the United States: JAMA Surg, 2015; 150(3); 252-59
5. Smith JM, Biggins SW, Haselby DG, Kidney, pancreas and liver allocation and distribution in the United States: Am J Transplant, 2012; 12(12); 3191-212
6. Maluf DG, Edwards EB, Kauffman HM, Utilization of extended donor criteria liver allograft: Is the elevated risk of failure independent of the model for end-stage liver disease score of the recipient?: Transplantation, 2006; 82(12); 1653-57
7. Bonney GK, Aldersley MA, Asthana S, Donor risk index and MELD interactions in predicting long-term graft survival: A single-centre experience: Transplantation, 2009; 87(12); 1858-63
8. Croome KP, Marotta P, Wall WJ, Should a lower quality organ go to the least sick patient? Model for end-stage liver disease score and donor risk index as predictors of early allograft dysfunction: Transplant Proc, 2012; 44(5); 1303-6
9. Schaubel DE, Sima CS, Goodrich NP, The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality: Am J Transplant, 2008; 8(2); 419-25
10. Amin MG, Wolf MP, TenBrook JA, Expanded criteria donor grafts for deceased donor liver transplantation under the MELD system: A decision analysis: Liver Transpl, 2004; 10(12); 1468-75
11. Schemmer P, Nickkholgh A, Hinz U, Extended donor criteria have no negative impact on early outcome after liver transplantation: A single-center multivariate analysis: Transplant Proc, 2007; 39(2); 529-34
12. Merion RM, When is a patient too well and when is a patient too sick for a liver transplant?: Liver Transpl, 2004; 10(10 Suppl 2); S69-73
13. Schaubel D, Sima CS, Goodrich N, Survival benefit of liver transplantation by MELD and Donor Risk Index: Am J Transplant, 2006; 6(1); 95
14. Verhoeven CJ, Farid WR, de Jonge J, Biomarkers to assess graft quality during conventional and machine preservation in liver transplantation: J Hepatol, 2014; 61(3); 672-84
15. Pine JK, Aldouri A, Young AL, Liver transplantation following donation after cardiac death: an analysis using matched pairs: Liver Transpl, 2009; 15(9); 1072-82
16. Gastaca M, Extended criteria donors in liver transplantation: Adapting donor quality and recipient: Transplant Proc, 2009; 41(3); 975-79
17. Nemes B, Gelley F, Zádori G, Outcome of liver transplantation based on donor graft quality and recipient status: Transplant Proc, 2010; 42(6); 2327-30
18. Wood NL, VanDerwerken DN, King EA, Life expectancy without a transplant for status 1A liver transplant candidates: Am J Transplant, 2022; 22(1); 274-78
19. Bruix J, Llovet JM, Two decades of advances in hepatocellular carcinoma research: Semin Liver Dis, 2010; 30(1); 1-2
20. Charpentier KP, Cheah YL, Machan JT, Intention to treat survival following liver transplantation for hepatocellular carcinoma within a donor service area: HPB (Oxford), 2008; 10(6); 412-15
21. Kitisin K, Packiam V, Steel J, Presentation and outcomes of hepatocellular carcinoma patients at a western centre: HPB (Oxford), 2011; 13(10); 712-22
22. Smith JO, Shiffman ML, Behnke M, Incidence of prolonged length of stay after orthotopic liver transplantation and its influence on outcomes: Liver Transpl, 2009; 15(3); 273-79
23. Cameron AM, Ghobrial RM, Yersiz H, Optimal utilization of donor grafts with extended criteria: A single-center experience in over 1000 liver transplants: Ann Surg, 2006; 243(6); 748-53 discussion 753–55
24. Luo X, Leanza J, Massie AB, MELD as a metric for survival benefit of liver transplantation: Am J Transplant, 2018; 18(5); 1231-37
25. Bhangui P, Vibert E, Majno P, Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: Living versus deceased donor transplantation: Hepatology, 2011; 53(5); 1570-79
26. Rana A, Riaz IB, Gruessner AC, Gruessner RW, Geographic inequity results in disparate mortality: A multivariate intent-to-treat analysis of liver transplant data: Clin Transplant, 2015; 29(6); 484-91
27. Hu Z, Li Z, Xiang J, Intent-to-treat analysis of liver transplant for hepatocellular carcinoma in the MELD era: impact of hepatitis C and advanced status: Dig Dis Sci, 2014; 59(12); 3062-72
28. Leise MD, Kim WR, Kremers WK, A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation: Gastroenterology, 2011; 140(7); 1952-60
29. Maluf DG, Edwards EB, Stravitz RT, Kauffman HM, Impact of the donor risk index on the outcome of hepatitis C virus-positive liver transplant recipients: Liver Transpl, 2009; 15(6); 592-99
Figures
 Figure 1. Study population exclusion criteria. A total of 72,918 patients who received liver transplants from June 18, 2013, to June 18, 2022 were assessed for eligibility. The figure displays exclusion criteria used to select the 9923 patients included in this analysis. StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: Stata Corp LLC.
Figure 1. Study population exclusion criteria. A total of 72,918 patients who received liver transplants from June 18, 2013, to June 18, 2022 were assessed for eligibility. The figure displays exclusion criteria used to select the 9923 patients included in this analysis. StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: Stata Corp LLC. Figure 2. Kaplan-Meier curve for survival in patients with MELD scores ≥35. (A) Patient survival shows significant difference for high- and normal-quality donors, compared with low-quality donors (log-rank, P<0.001 and P=0.02, respectively. (B) Graft survival shows significant differences for high- and normal-quality donors, compared with low-quality donors (log-rank, P<0.001 and P<0.001, respectively). StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.
Figure 2. Kaplan-Meier curve for survival in patients with MELD scores ≥35. (A) Patient survival shows significant difference for high- and normal-quality donors, compared with low-quality donors (log-rank, P<0.001 and P=0.02, respectively. (B) Graft survival shows significant differences for high- and normal-quality donors, compared with low-quality donors (log-rank, P<0.001 and P<0.001, respectively). StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC. Figure 3. Long-term (1 year) survivor patient mortality. Insignificant differences were found for high- and normal-quality donors, compared with low-quality donors (log-rank, P=0.19 and P=0.11, respectively). StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.
Figure 3. Long-term (1 year) survivor patient mortality. Insignificant differences were found for high- and normal-quality donors, compared with low-quality donors (log-rank, P=0.19 and P=0.11, respectively). StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC. Figure 4. Primary non-function by liver quality. Low-, normal-, and high-quality donors make up 2.52%, 2.13%, and 2.07% of patients, respectively, with MELD scores ≥35. Log-rank testing shows insignificant differences for high- and low-quality donors, compared with normal-quality donors. StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.
Figure 4. Primary non-function by liver quality. Low-, normal-, and high-quality donors make up 2.52%, 2.13%, and 2.07% of patients, respectively, with MELD scores ≥35. Log-rank testing shows insignificant differences for high- and low-quality donors, compared with normal-quality donors. StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC. Tables
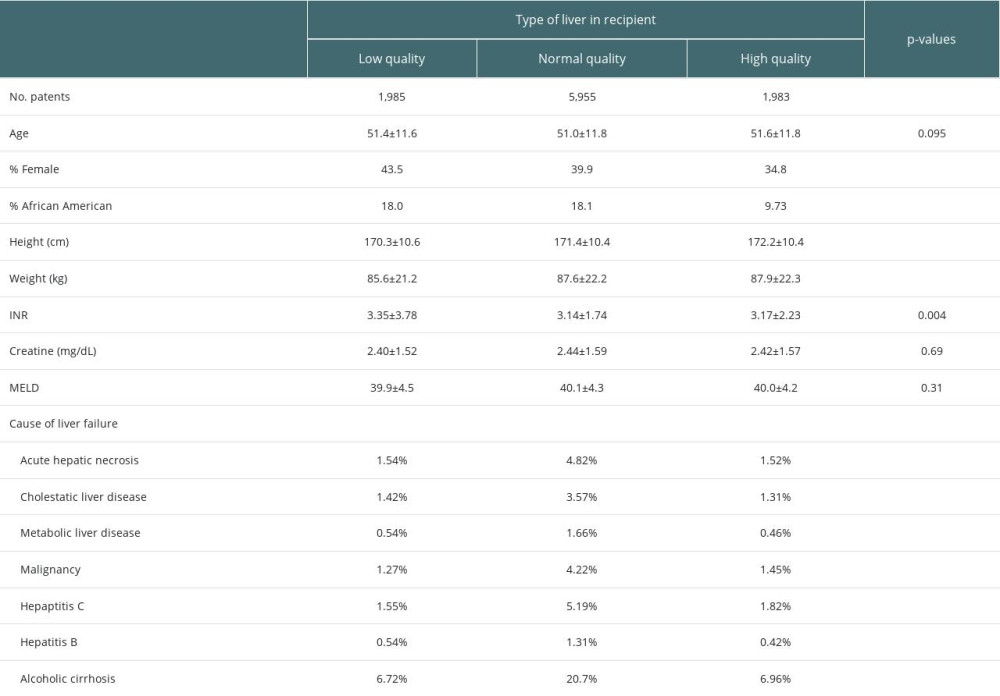 Table 1. Recipient demographic characteristics.
Table 1. Recipient demographic characteristics. Table 2. Donor and recipient risk factors considered in analyses.
Table 2. Donor and recipient risk factors considered in analyses.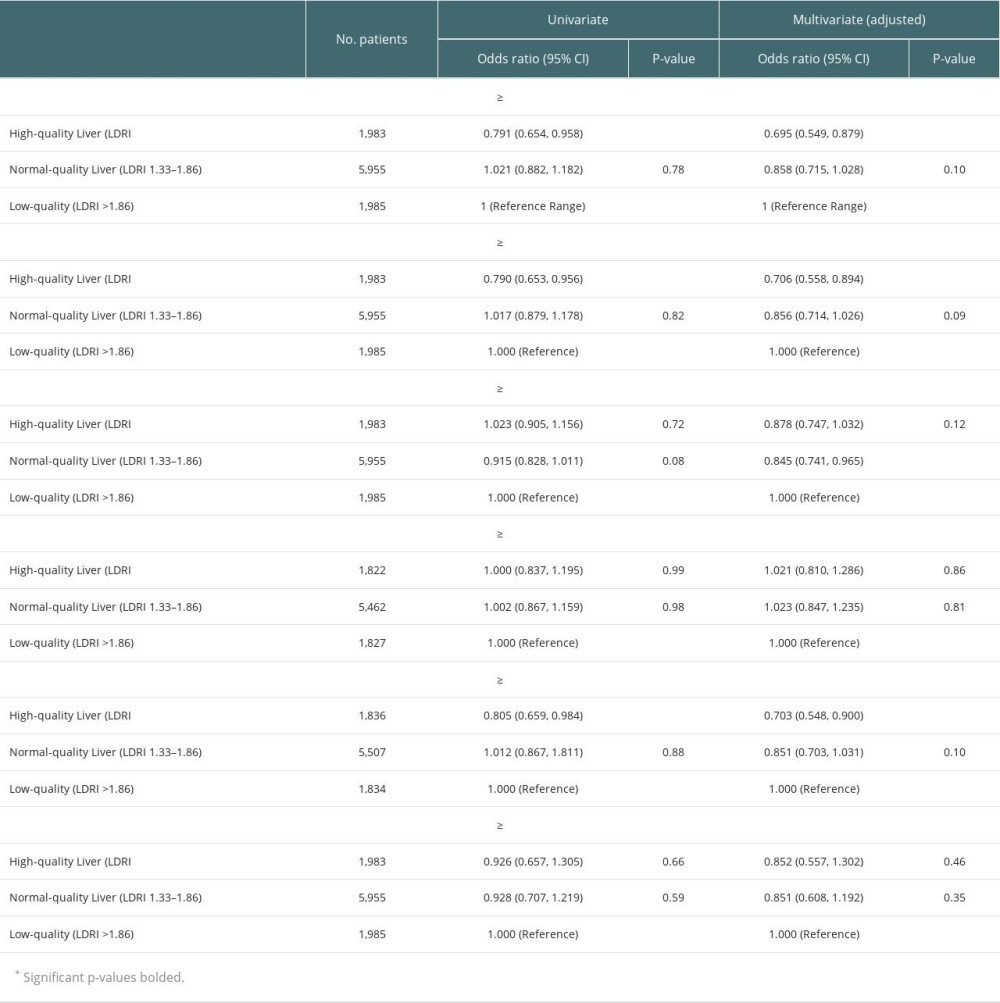 Table 3. Univariate and multivariate logistic regression of high- and normal-quality livers, compared with low-quality livers.
Table 3. Univariate and multivariate logistic regression of high- and normal-quality livers, compared with low-quality livers. Table 1. Recipient demographic characteristics.
Table 1. Recipient demographic characteristics. Table 2. Donor and recipient risk factors considered in analyses.
Table 2. Donor and recipient risk factors considered in analyses. Table 3. Univariate and multivariate logistic regression of high- and normal-quality livers, compared with low-quality livers.
Table 3. Univariate and multivariate logistic regression of high- and normal-quality livers, compared with low-quality livers. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








