19 December 2023: Original Paper
Allogeneic Hematopoietic Stem Cell Transplantation Can Improve Prognosis of Extramedullary Infiltration Positive t(8;21) Acute Myeloid Leukemia
Xiaokai Wang1BCDEF, Xuetong Xu2BCDF, Hao Zhang3CDF, Wei Zhou45BC, Dan Gong6BD, Chengying Zhu78CF, Dejun Zhou9ADFG, Guofeng Chen9ABCDEFG*DOI: 10.12659/AOT.942197
Ann Transplant 2023; 28:e942197
Abstract
BACKGROUND: In t(8;21) acute myeloid leukemia (AML), patients with extramedullary infiltration (EMI) tend to have worse survival outcomes than those without EMI. However, it is still unclear whether allogeneic hematopoietic stem cell transplantation (allo-HSCT) benefits EMI-positive t(8;21) AML patients.
MATERIAL AND METHODS: This study retrospectively enrolled 651 t(8;21) AML patients, and analyzed 51 patients with EMI at diagnosis. Among the 51 patients, 15 patients received allo-HSCT.
RESULTS: The incidence of EMI in t(8;21) AML was 10.0%, and the first complete remission rate was 78.5% in EMI-positive t(8;21) AML patients. The central nervous system was the most frequently involved site (29.4%), followed by bones (15.7%), and skin (9.8%). In terms of karyotype, 19 (37.3%) patients were t(8;21) alone, 12 (23.5%) had additional loss of a sex chromosome, and 5 (9.8%) had complex karyotype. Significantly better overall survival was observed in patients with allo-HSCT compared to patients without allo-HSCT in both multivariable models (HR=0.32; P=0.0122) and the Kaplan-Meier curves (P=0.0157).
CONCLUSIONS: Allo-HSCT improved the survival of EMI-positive t(8;21) AML.
Keywords: Hematopoiesis, Extramedullary, Hematopoietic Stem Cell Transplantation, Leukemia
Background
In patients with acute myeloid leukemia (AML), t(8;21)(q22;q22) is observed in up to 12% of total AML cases [1]. Compared with other types of AML, the incidence of extramedullary infiltration (EMI) was higher in t(8;21) AML [2]. Moreover, EMI was associated with poor prognosis in t(8;21) AML [3,4]. Hu et al [5] revealed that the complete remission (CR) rate (60% vs 78.4%,
Allogeneic hematopoietic stem cell transplant (allo-HSCT) is an established treatment modality with curative potential for AML [8]. Hu et al [9] showedthat EMI at diagnosis was associated with a high cumulative incidence of relapse, and allo-HSCT can improve the RFS (
Material and Methods
PATIENTS:
Data from 651 Chinese patients with t(8;21) AML were retrospectively collected from 15 AML study groups between 2002 and 2018. Data were obtained using special case report forms. The research was conducted in accordance with the Institutional Review Board guidelines of the participating study groups and the principles of the Declaration of Helsinki.
The criteria for patient exclusion were as follows: (1) 37 patients had no treatment information; (2) 549 patients were without detection of EMI at diagnosis; (3) 11 patients did not achieve CR1; (4) 3 patients were treated with auto-HSCT after CR1. The remaining 51 patients were included in the analysis for this study. Figure 1 provides details on study enrollment and Table 1 summarizes the baseline characteristics of 51 remaining patients in the study.
TREATMENT:
All the 51 remaining patients received 1–2 cycles of the standard ‘7+3’ induction chemotherapy, and achieved CR1 during induction therapy. The regimens of consolidation chemotherapy were either a single Ara-c or Ara-c combined with drugs such as daunorubicin, idarubicin, aclarubicin, mitoxantrone, homoharringtonine, pirarubicin, or fludarabine. The details of the therapeutic regimens have been reported previously [10,11]. Among the 51 remaining patients, 15 patients received allo-HSCT.
CONDITIONING REGIMENS:
Patients were given myeloablative or reduced-intensity conditioning regimens, as previously reported [12]. Myeloablative conditioning regimens included 0.8 mg/kg/6 h busulfan for 4 days, 60 mg/kg/day cyclophosphamide for 2 days, 50 mg/kg/day cyclophosphamide for 2 days, and 800–1000 cGy total body irradiation. Reduced-intensity conditioning regimens included 30 mg/m2/day fludarabine for 5 days, 3.2 mg/kg/day busulfan for 2 days, or reduced-dose TBI. Anti-thymocyte globulin (ATG) was used in patients who accepted haploidentical-related donor transplant or unrelated donor transplant.
GRAFT-VERSUS-HOST DISEASE (GVHD) PROPHYLAXIS STRATEGIES:
We used mycophenolate mofetil, cyclosporine, and methotrexate to prevent GVHD. Methylprednisolone was added in patients with grades II to IV aGVHD. Patients with extensive cGVHD were treated with prednisone alone or combined with mesenchymal stem cell. Patients with refractory aGVHD were given basiliximab. The details of GVHD prophylaxis strategies were shown in a previous article [13].
INFECTION PREVENTION AND SUPPORTIVE CARE:
Patients with agranulocytosis were given acyclovir and cotrimoxazole for prophylaxis. Red blood cell transfusions were administered in patients when hemoglobin levels <80 g/L, and platelets were used in patients when platelets count <10×109/L. Patients received recombinant human granulocyte macrophage colony stimulating factor after cell infusion. The infection prevention was mentioned in a previous article [13].
MINIMAL RESIDUAL DISEASE (MRD) MONITORING:
Minimal residual disease was monitored on days 30, 60, 90, and 180 after allo-HSCT.
DEFINITIONS OF EVENTS AND END POINTS:
Outcomes were evaluated by using the probability of the overall survival (pOS). The OS of the population was calculated from the date of diagnosis to the date of the last follow-up for alive patients or the date of death for any cause. Response criteria by the International Working Group (IWG) were considered to evaluate treatment response [14]. We defined CR as meeting all of the following response criteria for at least 4 weeks: bone marrow shows normal hematopoiesis, bone marrow blasts < 5%, no blasts with Auer rods or persistence of extramedullary disease, absolute neutrophil count >1×109/L, platelets ≥100×109/L, no residual evidence of extramedullary disease, and transfusion independent [14]. Non-relapse mortality was defined as any death in the first 28 days after transplantation or any death after day 28 in continuous remission.
STATISTICS:
The clinical characteristics were examined using the χ2 analysis or Fisher’s exact test for categorical variables. The pOS were estimated by the Kaplan-Meier method and compared using the log-rank test for univariate analysis and using the Cox regression analysis for multivariate analysis. Variables significant at
Results
From the 651 t(8;21) AML patients, 65 (10.0%) patients were EMI-positive at diagnosis. And among the 65 patients, 51 (78.5%) patients achieved CR1 after induce treatment. The remaining 51 patients were included in the study. The most frequently involved site was the central nervous system (CNS, 29.4%), followed by bones (15.7%), and skin (9.8%). Fourteen (27.5%) patients had multiple infiltration sites. The FLT3-ITD mutation was detected in 1 patient, but c-KIT mutation was identified in 12 patients. Out of the 51 cases, 19 (37.3%) had t(8;21) alone, 12 (23.5%) had additional loss of a sex chromosome, and 5 (9.8%) had complex karyotype.
Among the remaining 51 patients, 15 patients received allo-HSCT after CR1. We analyzed the characteristics of patients who received allo-HSCT or not. There were no significant differences in age, sex, white blood cells count (WBC), hemoglobin (Hb), platelets (PLT), bone marrow (BM) blasts, EMI sites, numbers of additional abnormal karyotype, type of karyotype, KIT mutation, WT1, or FLT3-ITD between the patients with and without allo-HSCT. The characteristics of patients are summarized in Table 1.
The median follow-up for all the 51 remaining patients was 21.3 months (95% CI; 19.2–35.9). The median OS for the allo-HSCT negative group was 19.7 months (95% CI; 14.7–28.3), whereas the median OS for the allo-HSCT positive group was not reached (Figure 2). Moreover, patients with allo-HSCT had lower mortality (3-year pOS, 52.98%) compared to patients without allo-HSCT (3-year pOS, 21.63%,
Data from 15 patients who received allo-HSCT are shown in Table 4. Disease status at transplantation was: 11 cases (73.3%) that were in CR1, 2 cases (13.3%) that were in second complete remission (CR2), and 2 cases (13.3%) that had no remission (NR) of disease. Among them, 7 cases were matched sibling transplantation, 5 cases were matched unrelated transplantation, and 3 cases were haploidentical transplantation. Among the 3 patients with CNS extramedullary infiltration, 2 (66.7%) experienced post-transplant relapse. The post-transplant relapse incidence rate for all 15 patients who received allo-HSCT was 13.3%, and the non-relapse mortality rate was 0%.
Discussion
In this study, the incidence of EMI in t(8;21) AML was 10.0%. It was similar to other studies, with the incidence of 9.5–10.2% [2,6]. In our study, the CR1 rate was 78.5% in EMI-positive t(8;21) AML patients. Moreover, the CR1 rate was 94.1% and 50% in study of Byrd et al [2] and Park et al [6], respectively. In our study, 51 t(8;21) AML patients with EMI remained at diagnosis. The most frequently involved site was the CNS, which was consistent with the study of Park et al [6]. Moreover, in an article by Byrd et al [2] there were 4 patients with D-spine involvement, 1 patient with sacrum involvement, and 1 patient with meningeal involvement among 8 patients of EMI-positive t(8;21), which was comparable to our data.
Studies showed that EMI is associated with poorer prognosis in t(8;21) AML [15,16]. Although patients with EMI AML and high-risk cytogenetics are candidates for allo-HSCT consensually [17], few report have shown the effects of allo-HSCT in EMI-positive t(8;21) AML patients. In patients with EMI, the median OS of the patients who underwent HSCT was 40.6 months, compared with 9.4 months in patients who did not (
In addition to allo-HSCT, there are other newer treatment options for EMI-positive AML, such as intensive chemotherapy, donor lymphocyte infusion, radiation therapy, CAR T-cell therapy, or gemtuzumab ozogamicin [21–23]. Unfortunately, our study could not compare allo-HSCT with other treatment methods in EMI-positive t(8;21) AML because the number of patients receiving other newer treatment was insufficient. This study has other limitations. The data were collected retrospectively, the patient population was heterogeneous, and patients were treated according to a variety of protocols with different treatment strategies. Despite these limitations, we believe that this study described the clinical characteristics, identified risk factors, and gave a reliable estimate of the effect of allo-HSCT on OS in patients with EMI-positive t(8;21) AML.
Conclusions
In EMI-positive t(8;21) AML, the most involved site was the CNS, followed by bones. Allo-HSCT may improve OS in EMI-positive t(8;21) AML patients.
Figures
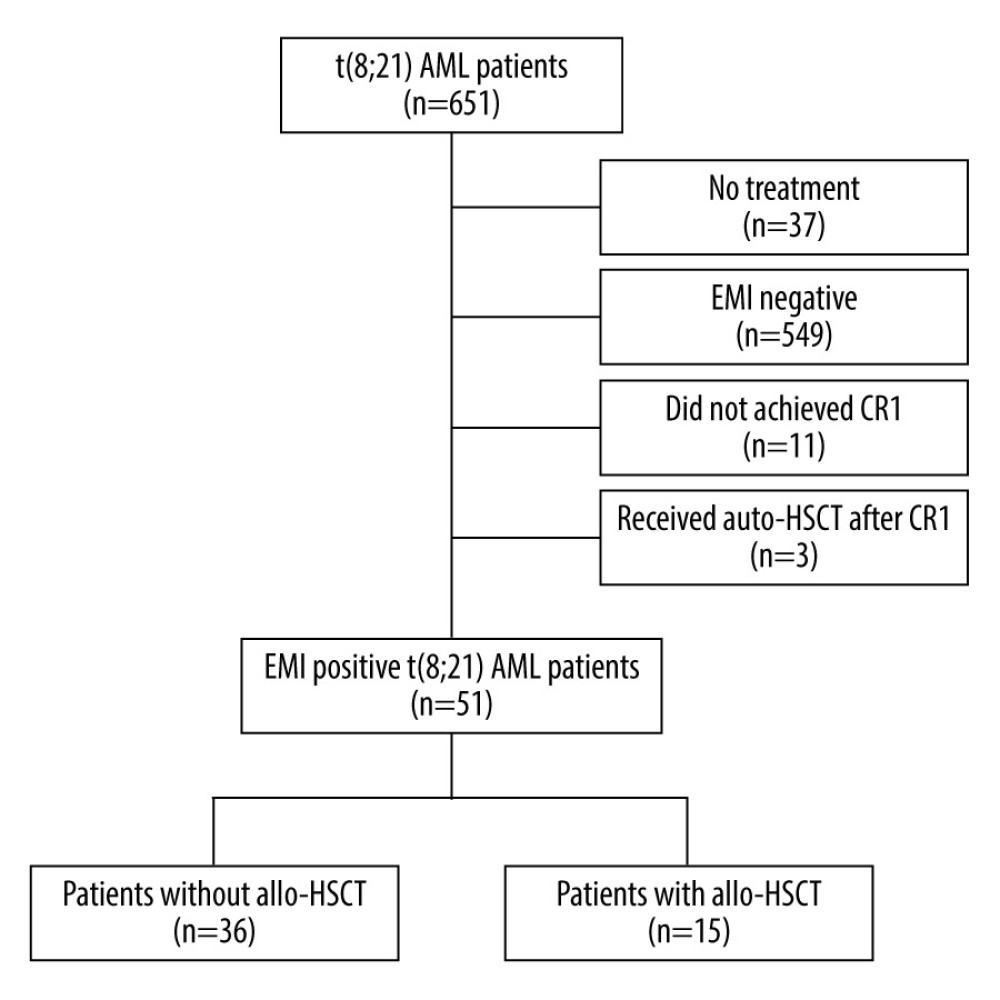 Figure 1. Study enrollment chart. AML – acute myeloid leukemia; EMI – extramedullary infiltration; CR1 – first complete remission; auto-HSCT – autologous hematopoietic stem cell transplantation; allo-HSCT – allogeneic hematopoietic stem cell transplantation. Figure were performed using the PowerPoint (Microsoft® PowerPoint® 2016, 16.0.4266.1001).
Figure 1. Study enrollment chart. AML – acute myeloid leukemia; EMI – extramedullary infiltration; CR1 – first complete remission; auto-HSCT – autologous hematopoietic stem cell transplantation; allo-HSCT – allogeneic hematopoietic stem cell transplantation. Figure were performed using the PowerPoint (Microsoft® PowerPoint® 2016, 16.0.4266.1001). 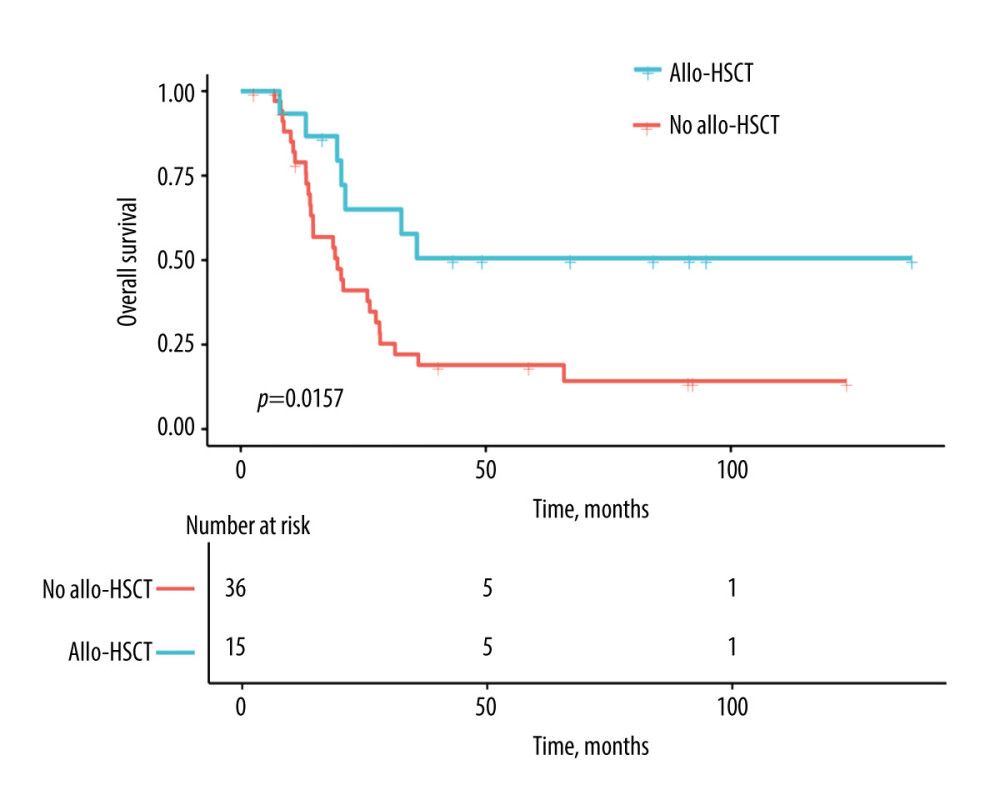 Figure 2. Estimates for EMI-positive t(8;21) AML patients with or without allo-HSCT. Allo-HSCT – allogeneic hematopoietic stem cell transplantation. Figure were performed using the EmpowerStats (http://www.empowerstats.com, X & Y Solutions, Inc., Boston, MA).
Figure 2. Estimates for EMI-positive t(8;21) AML patients with or without allo-HSCT. Allo-HSCT – allogeneic hematopoietic stem cell transplantation. Figure were performed using the EmpowerStats (http://www.empowerstats.com, X & Y Solutions, Inc., Boston, MA). Tables
Table 1. Clinical characteristics in t(8;21) AML patients with EMI at diagnosis.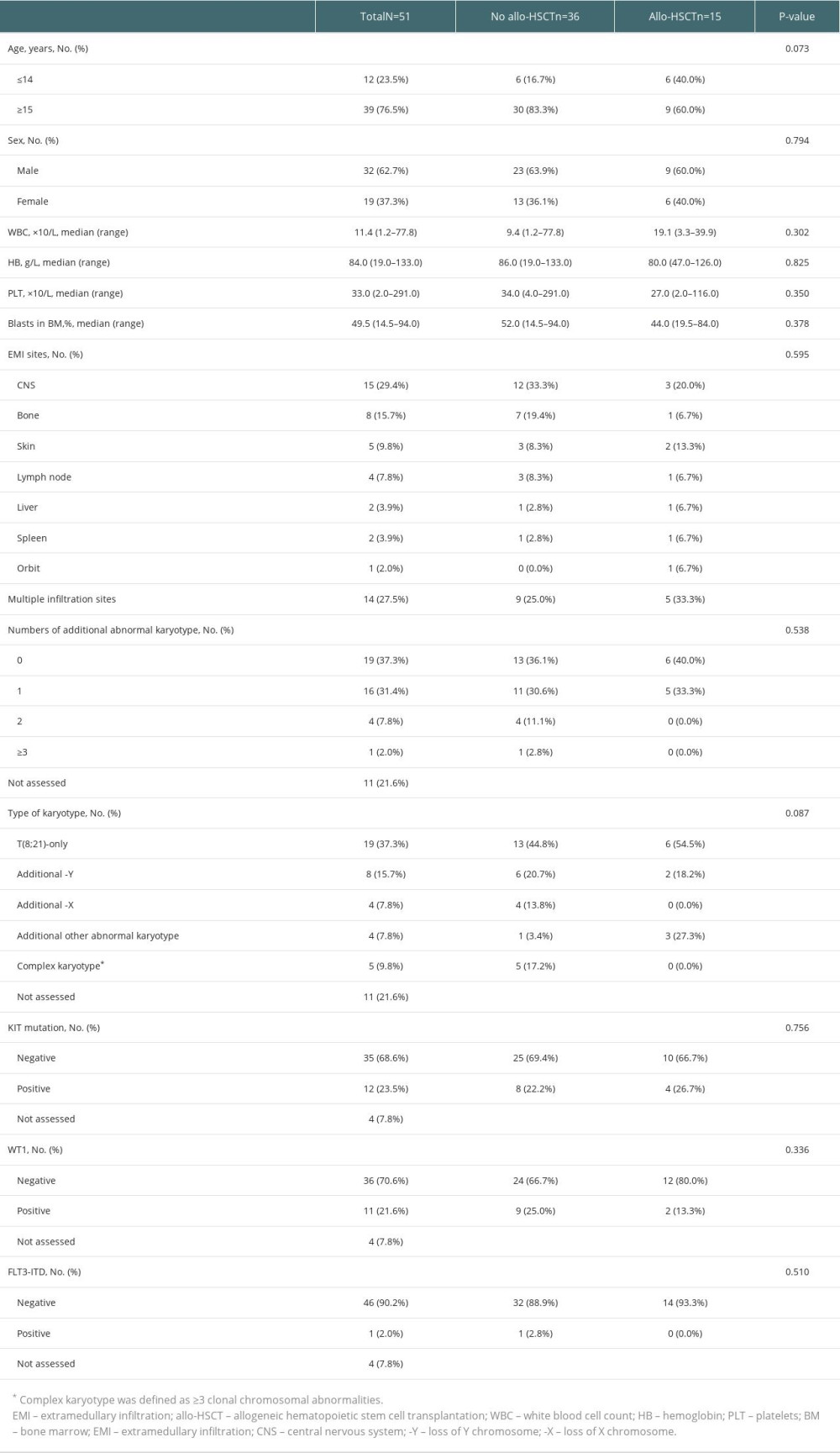 Table 2. Univariable models of pOS in t(8;21) AML patients with EMI at diagnosis.
Table 2. Univariable models of pOS in t(8;21) AML patients with EMI at diagnosis.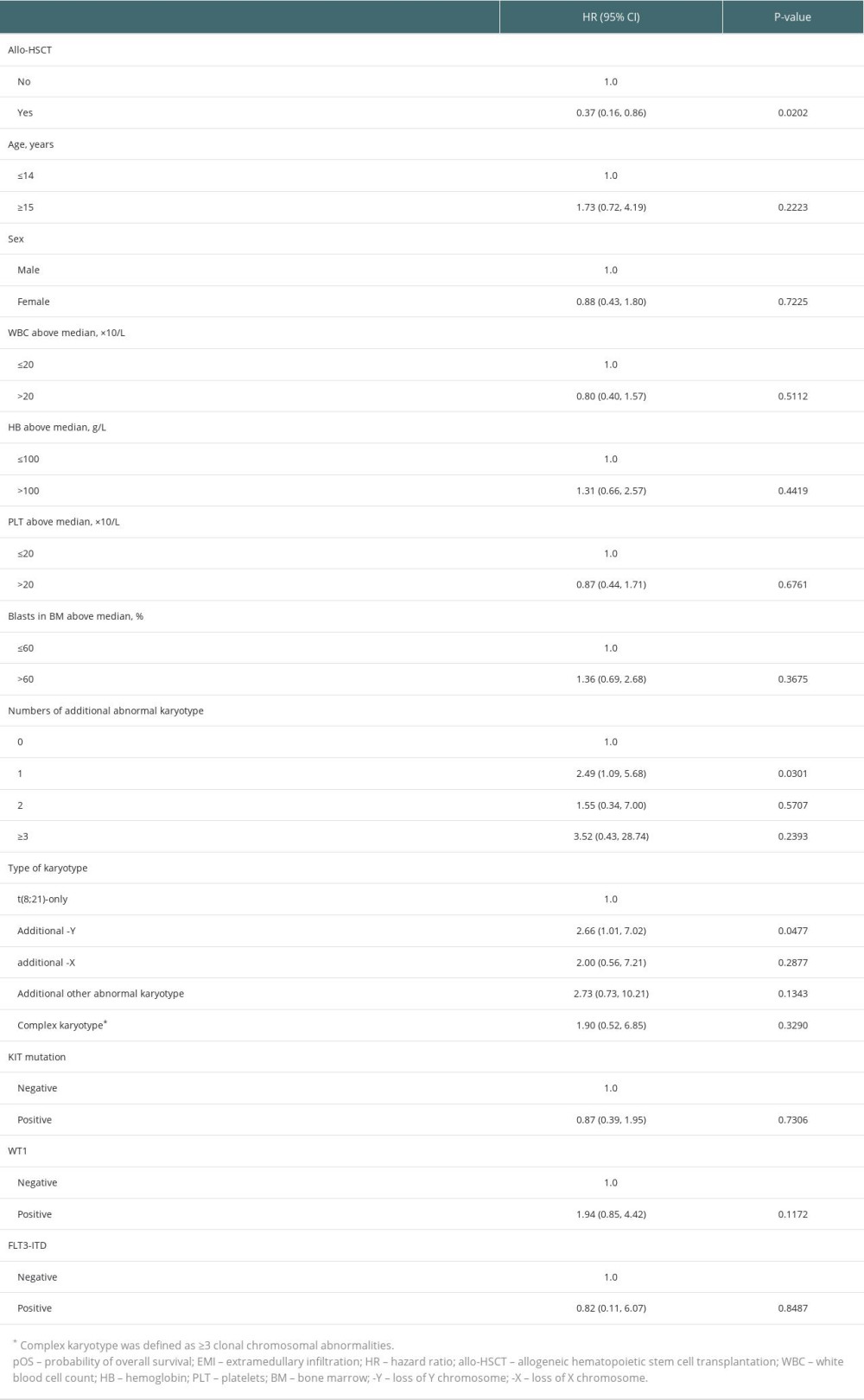 Table 3. Multivariable models of pOS in t(8;21) AML patients with EMI at diagnosis.
Table 3. Multivariable models of pOS in t(8;21) AML patients with EMI at diagnosis.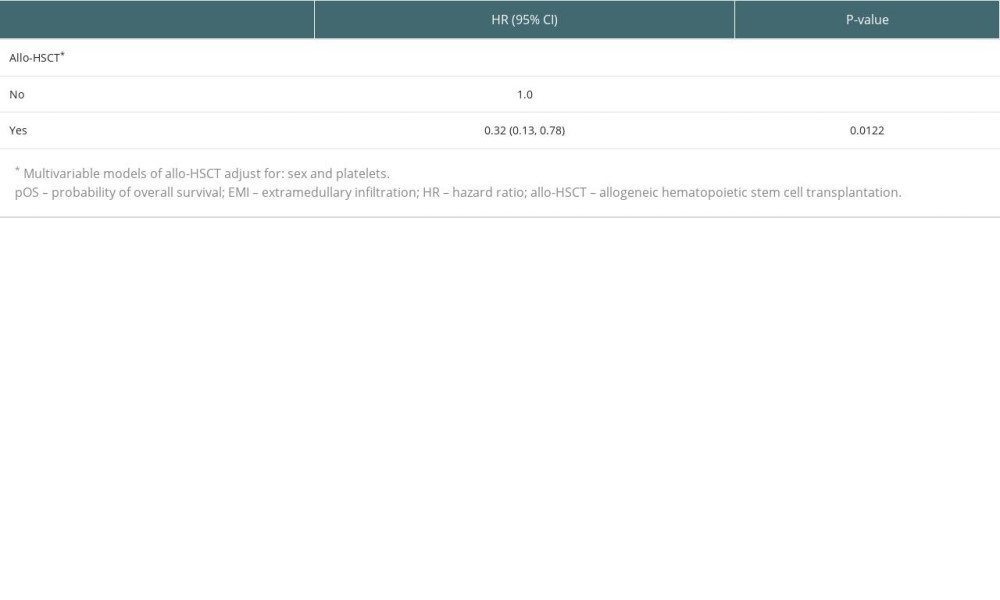 Table 4. Characteristics of 15 patients who received allo-HSCT.
Table 4. Characteristics of 15 patients who received allo-HSCT.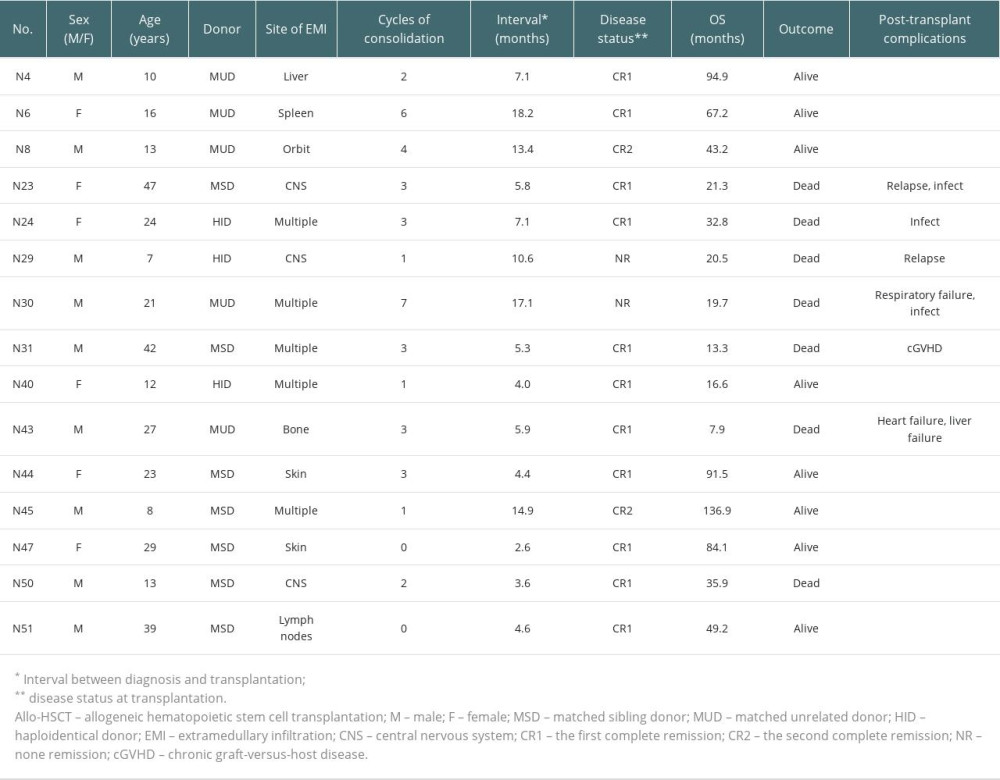
References
1. Lin P, Chen L, Luthra R, Konoplev SN, Acute myeloid leukemia harboring t(8;21)(q22;q22): A heterogeneous disease with poor outcome in a subset of patients unrelated to secondary cytogenetic aberrations: Mod Pathol, 2008; 21(8); 1029-36
2. Byrd JC, Weiss RB, Arthur DC, Extramedullary leukemia adversely affects hematologic complete remission rate and overall survival in patients with t(8;21)(q22;q22): Results from Cancer and Leukemia Group B 8461: J Clin Oncol, 1997; 15(2); 466-75
3. Baul SN, Baveja A, Mandal PK, A glimpse into translocation (8;21) in acute myeloid leukemia: Profile and therapeutic outcomes from a tertiary care hematology center from East India: J Hematol Allied Sci, 2022; 2; 85-90
4. Hu GH, Lu AD, Jia YP, Prognostic impact of extramedullary infiltration in pediatric low-risk acute myeloid leukemia: A retrospective single-center study over 10 years: Clin Lymphoma Myeloma Leuk, 2020; 20(11); e813-e20
5. Hu G, Lu A, Wu J, Characteristics and prognosis of pediatric myeloid sarcoma in the cytogenetic context of t(8;21): Pediatr Hematol Oncol, 2021; 38(1); 14-24
6. Park SS, Yoon JH, Kim HJ, Characteristics and survival outcome analysis of extramedullary involvement in adult patients with t(8;21) acute myeloid leukemia: Clin Lymphoma Myeloma Leuk, 2017; 17(1); 38-45e2
7. Gong D, Li W, Hu LD, Comparison of clinical efficacy of cytarabine with different regimens in postremission consolidation therapy for adult t(8;21) AML patients: A multicenter retrospective study in China: Acta Haematol, 2016; 136(4); 201-9
8. Magenau J, Couriel DR, Hematopoietic stem cell transplantation for acute myeloid leukemia: To whom, when, and how: Curr Oncol Rep, 2013; 15(5); 436-44
9. Hu GH, Cheng YF, Lu AD, Allogeneic hematopoietic stem cell transplantation can improve the prognosis of high-risk pediatric t(8;21) acute myeloid leukemia in first remission based on MRD-guided treatment: BMC Cancer, 2020; 20(1); 553
10. Chen G, Zhou W, Gong D, Loss of X chromosome predicts favorable prognosis in female patients with t(8;21) acute myeloid leukemia: Leuk Lymphoma, 2020; 61(5); 1168-77
11. Zhou W, Chen G, Gong D, Loss of the Y chromosome predicts a high relapse risk in younger adult male patients with t(8;21) acute myeloid leukemia on high-dose cytarabine consolidation therapy: A retrospective multicenter study: Leuk Lymphoma, 2020; 61(4); 820-30
12. Zhou W, Chen G, Gong D, Risk factors for post-transplant relapse and survival in younger adult patients with t(8;21)(q22;q22) acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation: A multicenter retrospective study: Front Oncol, 2023; 13; 1138853
13. Zhu CY, Chen GF, Zhou W, Outcome and prognostic factors of high-risk acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: Ann Transplant, 2019; 24; 328-40
14. Cheson BD, Bennett JM, Kopecky KJInternational Working Group for Diagnosis, Standardization of Response Criteria, reatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia, Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia: J Clin Oncol, 2003; 21(24); 4642-49 Erratum in: J Clin Oncol. 2004;22(3):576
15. Tallman MS, Hakimian D, Shaw JM, Granulocytic sarcoma is associated with the 8;21 translocation in acute myeloid leukemia: J Clin Oncol, 1993; 11(4); 690-97
16. Fianchi L, Quattrone M, Criscuolo M, Extramedullary involvement in acute myeloid leukemia. A single center ten years’ experience: Mediterr J Hematol Infect Dis, 2021; 13(1); e2021030
17. Cornelissen JJ, Gratwohl A, Schlenk RF, The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: An integrated-risk adapted approach: Nat Rev Clin Oncol, 2012; 9(10); 579-90
18. Ganzel C, Manola J, Douer D, Extramedullary disease in adult acute myeloid leukemia is common but lacks independent significance: Analysis of patients in ECOG-ACRIN Cancer Research Group Trials, 1980–2008: J Clin Oncol, 2016; 34(29); 3544-53 Erratum in: J Clin Oncol. 2017;35(2): 263
19. Eckardt JN, Stölzel F, Kunadt D, Molecular profiling and clinical implications of patients with acute myeloid leukemia and extramedullary manifestations: J Hematol Oncol, 2022; 15(1); 60
20. Shan M, Lu Y, Yang M, Characteristics and transplant outcome of myeloid sarcoma: A single-institute study: Int J Hematol, 2021; 113(5); 682-92
21. Piccaluga PP, Martinelli G, Rondoni M, Gemtuzumab ozogamicin for relapsed and refractory acute myeloid leukemia and myeloid sarcomas: Leuk Lymphoma, 2004; 45(9); 1791-95
22. Ando T, Mitani N, Matsunaga K, Gemtuzumab ozogamicin therapy for isolated extramedullary AML relapse after allogeneic hematopoietic stem-cell transplantation: Tohoku J Exp Med, 2010; 220(2); 121-26
23. Bakst RL, Tallman MS, Douer D, Yahalom J, How I treat extramedullary acute myeloid leukemia: Blood, 2011; 118(14); 3785-93
Figures
 Figure 1. Study enrollment chart. AML – acute myeloid leukemia; EMI – extramedullary infiltration; CR1 – first complete remission; auto-HSCT – autologous hematopoietic stem cell transplantation; allo-HSCT – allogeneic hematopoietic stem cell transplantation. Figure were performed using the PowerPoint (Microsoft® PowerPoint® 2016, 16.0.4266.1001).
Figure 1. Study enrollment chart. AML – acute myeloid leukemia; EMI – extramedullary infiltration; CR1 – first complete remission; auto-HSCT – autologous hematopoietic stem cell transplantation; allo-HSCT – allogeneic hematopoietic stem cell transplantation. Figure were performed using the PowerPoint (Microsoft® PowerPoint® 2016, 16.0.4266.1001). Figure 2. Estimates for EMI-positive t(8;21) AML patients with or without allo-HSCT. Allo-HSCT – allogeneic hematopoietic stem cell transplantation. Figure were performed using the EmpowerStats (http://www.empowerstats.com, X & Y Solutions, Inc., Boston, MA).
Figure 2. Estimates for EMI-positive t(8;21) AML patients with or without allo-HSCT. Allo-HSCT – allogeneic hematopoietic stem cell transplantation. Figure were performed using the EmpowerStats (http://www.empowerstats.com, X & Y Solutions, Inc., Boston, MA). Tables
 Table 1. Clinical characteristics in t(8;21) AML patients with EMI at diagnosis.
Table 1. Clinical characteristics in t(8;21) AML patients with EMI at diagnosis. Table 2. Univariable models of pOS in t(8;21) AML patients with EMI at diagnosis.
Table 2. Univariable models of pOS in t(8;21) AML patients with EMI at diagnosis. Table 3. Multivariable models of pOS in t(8;21) AML patients with EMI at diagnosis.
Table 3. Multivariable models of pOS in t(8;21) AML patients with EMI at diagnosis. Table 4. Characteristics of 15 patients who received allo-HSCT.
Table 4. Characteristics of 15 patients who received allo-HSCT. Table 1. Clinical characteristics in t(8;21) AML patients with EMI at diagnosis.
Table 1. Clinical characteristics in t(8;21) AML patients with EMI at diagnosis. Table 2. Univariable models of pOS in t(8;21) AML patients with EMI at diagnosis.
Table 2. Univariable models of pOS in t(8;21) AML patients with EMI at diagnosis. Table 3. Multivariable models of pOS in t(8;21) AML patients with EMI at diagnosis.
Table 3. Multivariable models of pOS in t(8;21) AML patients with EMI at diagnosis. Table 4. Characteristics of 15 patients who received allo-HSCT.
Table 4. Characteristics of 15 patients who received allo-HSCT. In Press
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
03 Apr 2024 : Review article
Alternative Therapies in Transplantology as a Promising Perspective in MedicineAnn Transplant In Press; DOI: 10.12659/AOT.943387
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








