06 February 2024: Original Paper
Immunoprotective Effect of Liver Allograft on Patients with Combined Liver and Kidney Transplantation
Joon-Young KimDOI: 10.12659/AOT.942763
Ann Transplant 2024; 29:e942763
Abstract
BACKGROUND: Simultaneous liver-kidney transplantation (SLKT) and kidney transplantation (KT) after liver transplantation (LT) provide potential treatment options for patients with end-stage liver and kidney disease. There is increasing attention being given to liver-kidney transplantation (LTKT), particularly regarding the immune-protective effects of the liver graft. This retrospective, single-center, observational study aimed to evaluate the clinical outcomes of KT in LTKT patients – either SLKT or KT after LT (KALT) – compared to KT alone (KTA).
MATERIAL AND METHODS: We included patients who underwent KT between January 2005 and December 2020, comprising a total of 4312 patients divided into KTA (n=4268) and LTKT (n=44) groups. The LTKT group included 11 SLKT and 33 KALT patients. To balance the difference in sample sizes between the 2 groups, we performed 3: 1 propensity score matching (PSM).
RESULTS: There was no significant difference in graft survival between the groups. However, the LTKT group exhibited significantly superior rejection-free survival compared to the KTA group (P<0.001). Although the difference in the rejection-free graft survival rate disappeared (P=0.081) following PSM, a significant difference was observed when the LTKT group was subdivided into SLKT and KALT groups (P=0.047). The SLKT group showed no rejection episodes during the follow-up period, while the KALT group did not exhibited significant difference in the presence of rejection compared to the KTA group.
CONCLUSIONS: These findings suggest that SLKT may have a protective effect against rejection compared to KTA, highlighting a potential immunoprotective role of liver grafts on kidney grafts.
Keywords: Kidney Transplantation, Graft Rejection, Liver Transplantation
Background
Liver transplantation (LT) is a life-saving procedure for patients with end-stage liver disease, many of whom also suffer from renal dysfunction [1]. Approximately 20–30% of individuals with cirrhosis experience renal insufficiency, primarily due to glomerulonephritis associated with chronic viral or alcoholic hepatitis [2]. Moreover, the occurrence of acute renal failure after liver transplantation is reported to be as high as 70%, with 7% of instances necessitating dialysis, thereby exacerbating patient mortality rates and decreasing the survival rate of the transplanted liver graft [3]. Higher mortality rates for simultaneous liver-kidney transplantation (SLKT) were observed in the early 2000s [4]; however, it has emerged as a promising treatment option recently, with 10% to 20% of LT in the United States now being performed in conjunction with kidney transplantation (KT) [5]. Even if liver transplantation is successfully performed, long-term exposure to calcineurin inhibitors increases the risk of developing chronic renal failure, especially in patients with borderline renal function. Additionally, pre-existing renal diseases such as hypertension, diabetes, and glomerulonephritis further contribute to long-term chronic renal failure, leading to an increasing number of patients requiring kidney transplantation after liver transplantation (KALT) [6].
Since implementing the Model for End-Stage Liver Disease (MELD) allocation policy in 2002, patients who undergo liver transplants have been experiencing a more significant burden of renal dysfunction, as serum creatinine is a significant factor in the MELD equation [7]. The consideration of serum creatinine means that a subset of these patients may need a KT as well as an LT, resulting in an increase in the number of candidates for SLKT. Nonetheless, the presence of recurrent early complications remains a substantial barrier to the implementation of SLKT. Despite ongoing efforts to reduce surgical complications in SLKT, patients undergoing SLKT still experience a higher mortality rate compared to patients receiving KTA and KALT [8,9]. This higher mortality rate is primarily attributable to surgical complications in the first year following transplantation. However, SLKT patients demonstrate superior rejection-free graft survival, indicating an immune-protective effect of the liver graft and its potential role in improving long-term outcomes for the kidney graft [8,9].
Clinical evidence has provided significant support for the immune-protective impacts of liver allografts on kidney allografts in recipients of SLKT compared to those who undergo kidney transplantation alone (KTA) [4,6,10–15]. The phenomenon known as the sponge effect of a liver graft has been postulated to play a crucial role in the sequestration of circulating anti-human leukocyte antigen (HLA) antibodies. However, there is currently a lack of large-scale single-center studies reporting the clinical outcomes of KALT [6,12,13,15–17]. This study aimed to evaluate and compare the outcomes of patients who underwent SLKT or KALT with those who underwent KTA by utilizing propensity score matching. In particular, by comparing the differences in rejection-free kidney graft survival between the groups, our goal was to understand and substantiate the potential immune-protective effect of liver grafts on kidney grafts.
Material and Methods
PATIENTS:
This study was a retrospective, observational analysis conducted at a single center, encompassing a cohort of patients who underwent KT from January 2005 through December 2020. We excluded patients who also received a pancreas (n=216) or heart (n=12) transplant concurrently. Consequently, a total of 4312 patients were considered for our investigation. These patients were classified into 2 primary groups: the KTA (n=4268) and LTKT (n=44) groups. Within the LTKT group, 11 patients underwent SLKT, while 33 patients had a KALT. All individuals in the cohort of SLKT received transplants from a single deceased donor for both the liver and kidney. However, in the KALT group, the liver and kidney grafts were obtained from different donors. Our study utilized data from the Asan Medical Center (AMC) registry in Seoul, Korea. The database undergoes a yearly update via a comprehensive evaluation of the medical records pertaining to patients who have undergone KT. The Institutional Review Board at the AMC (AMC IRB number 2022-0737) granted approval for the study’s protocols. The Institutional Review Board (IRB) waived the requirement for informed consent because this was a retrospective study classified as Level 1, with minimal associated risk. We accessed the data for research purposes from June 1, 2022, to February 28, 2023. To maintain participant anonymity, the authors did not access any personally identifying information during the data collection procedure. For the objectives of this study, only the necessary de-identified medical information was retrieved. The present study adhered to the ethical principles outlined in the World Medical Association Declaration of Helsinki.
IMMUNOSUPPRESSION:
This study implemented immunosuppressive therapies, including those for SLKT, in accordance with the standard protocols for KT. According to immunological risk, either basiliximab, an anti-IL-2 receptor antibody, or anti-thymocyte globulin (ATG) was employed for induction therapy. We employed calcineurin inhibitors, corticosteroids, and mycophenolic acid for maintenance therapy. Tacrolimus target trough levels in the early postoperative phase were 7–10 ng/ml and 100–150 ng/mL, respectively. However, after the first postoperative year, these target concentrations for tacrolimus and cyclosporin were gradually decreased to 3–6 ng/mL and 50–100 g/L, respectively. For ABO-incompatible (ABOi) and crossmatch (XM)-positive KT, a single dose of rituximab (200–500 mg) was given 1–2 weeks prior to plasmapheresis, with or without intravenous immunoglobulin.
DEFINITIONS:
The time from transplantation to the recipient’s death was defined as patient survival (PS), and the time from transplantation to the patient’s return to dialysis, re-transplantation, or last follow-up with a functional graft was classified as graft survival (GS). We also examined death-censored GS (DCGS), which involved censoring data if a patient died with a functional transplant. The interval between a kidney transplant and the incidence of an acute rejection (AR), which was determined by pathology, was called rejection-free GS. The diagnosis of AR was made based on pathological examination following the Banff criteria [18]. Additionally, C4d staining was conducted on all tissue samples. In this study, all references to AR pertain to kidney grafts only, not liver grafts. In our standard practice, we only conduct indication biopsies, and routine protocol biopsies were not performed.
STATISTICAL ANALYSIS:
The chi-squared test or Fisher’s exact test was employed in our study to compare categorical variables, while Student’s t-test was utilized for continuous variables, as deemed appropriate. The researchers employed Kaplan-Meier analysis to compute cumulative survival and conducted group comparisons using a log-rank test. Univariate and multivariate Cox proportional hazard regression analyses were used to identify risk factors contributing to rejection-free graft survival, with the results presented as hazard ratios (HRs). Variables with a P-value of less than 0.1 were incorporated into the multivariate analysis, which was conducted using the forward conditional method. The statistical analyses were performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA). To address the issue of differences in group sizes, the researchers utilized propensity score matching (PSM) in a 3: 1 ratio, employing the Statistical Analysis System (SAS®, SAS Institute, Inc., Cary, NC, USA) for this purpose. We included recipient variables in our PSM that have clinical relevance to post-transplant AR. These included age, sex, diabetes mellitus (DM), hypertension, hepatitis virus infection, body mass index (BMI), ABOi KT, FXCM KT, HLA-A, B, DR mismatches (MM), panel-reactive antibody (PRA) level, type of donor (deceased or living), and calcineurin inhibitors. The matching process was iterated until achieving the optimal matching of all covariates, ensuring that the standardized mean differences (SMD) were below 0.1. A significance level of 0.05 was chosen, and a P-value below this threshold was considered statistically significant.
Results
PATIENT DEMOGRAPHICS:
Table 1 presents the baseline and clinical characteristics of the 4312 study patients. Statistically significant differences between the groups were observed in the proportion of female patients (42.3% vs 15.9%, P<0.001), the prevalence of hepatitis B virus (HBV) (3.1% vs 27.3%, P<0.001), and the incidence of hypertension (84.5% vs 72.7%, P=0.033). Furthermore, a trend toward a higher rate of DM was noted in the LTKT group (23.9% vs 36.4%, P=0.053). There were no significant differences between the 2 groups for any other factors, such as age, body mass index, ABO incompatibility, positive FXCM, HLA-A, B, DR MM, PRA class I, PRA class II, type of donor, or type of calcineurin inhibitor and induction agent utilized.
POSTOPERATIVE CLINICAL OUTCOMES:
A summary of the postoperative clinical outcomes seen in 11 individuals who had SLKT is shown in Table 2. The average length of stay for these patients in the intensive care unit (ICU) was 14.8 days, ranging from 0 to 40 days. Five of these patients had delayed graft function (DGF), which is defined by the need for hemodialysis. Graft function was not recovered in 1 patient, leading to graft failure. Three cases of postoperative hemorrhage required surgical intervention. The range of the patients’ MELD scores was 21 to 33. The one-year PS rate showed no significant difference between the KTA and LTKT groups (1.7%, n=72 vs 4.5%, n=2; P-value=0.15).
Long-term kidney graft survival was analyzed using Kaplan-Meier analysis (Figure 1). This study did not find a statistically significant difference in the overall GS (P=0.068) or DCGS (P=0.151). However, for rejection-free GS, a significant difference was noted between the LTKT and the KTA groups (P<0.001) (Figure 2). A statistically significant difference was observed when the LTKT group was divided into SLKT and KALT groups (P=0.012). Notably, no AR occurred in the SLKT group during the follow-up period, while the KALT group did not show a significant difference compared to the KTA group (P=0.10). Thus, the reason for the significantly better rejection-free GS in the LTKT group is attributed to the absence of rejection in the SLKT group. The LTKT group exhibited a significantly lower overall rejection rate of 6.8% compared to 22.8% in the KTA group (P=0.012). However, both groups showed similar 1-year rejection rates (P=0.43). and distribution of rejection types, with acute cellular rejection at 4.5% for LTKT and 8.9% for KTA, and acute antibody-mediated rejection at 6.8% for LTKT and 13.7% for KTA, without any significant differences (P=0.49) (Supplementary Table 1).
In Table 3, we identify risk factors associated with acute rejection post-transplantation. Several variables demonstrated statistical significance in the univariate analysis examining risk factors for AR following transplantation. These included age, BMI, tacrolimus vs cyclosporin, flow cytometry (FC) XM positivity, HLA-A, B, DR MM, PRA class I and II, living vs deceased donor, and the LTKT vs KT group. Only variables from the univariate analysis with a P-value of less than 0.1 were included in the subsequent multivariate analysis. The results of the multivariate analysis indicate that age (HR=0.83, 95% CI 0.78–0.81, P<0.001), BMI (HR=1.01, 95% CI 1.00–1.01, P=0.004), tacrolimus use in comparison to cyclosporin (HR=0.56, 95% CI 0.46–0.64, P<0.001), and positive FCXM (HR=1.39, 95% CI 1.07–1.82, P=0.016). After adjusting for possible confounders, the LTKT group showed a protective effect against AR, with a hazard ratio of 0.27 (95% CI: 0.09–0.83; P=0.023).
PROPENSITY SCORE MATCHING ANALYSIS:
Table 4 presents a comparative analysis of the cohort of patients who underwent KTA, comprising 132 individuals, and the LTKT group, comprising 44 individuals. Both groups were matched using a propensity score to reduce bias from the non-randomized study design. The SMD values, which are all less than 0.1, suggest that after propensity score matching, the 2 groups were reasonably balanced regarding their baseline and clinical characteristics.
Table 5 presents the impact of LTKT on the rejection-free GS of kidney grafts in both unadjusted and propensity score-matched conditions. The statistical significance of the HR for rejection-free GS was 0.27 (95% CI: 0.09–0.83; P=0.023) prior to conducting the PSM analysis. However, after PSM, to account for possible confounding variables, the HR increased slightly to 0.36 (95% CI: 0.11–1.19) and was no longer statistically significant (P=0.095). This suggests that while there may be a protective effect of LTKT against graft rejection, the effect is not statistically significant when controlling for other factors.
LONG-TERM CLINICAL OUTCOMES AFTER PSM:
Following PSM, the Kaplan-Meier analysis revealed no statistically significant disparities in the overall GS (P=0.052) or DCGS (P=0.109) (Figure 3). Upon examination of the rejection-free GS, no significant distinction (P=0.081) was observed between the cohorts who underwent LTKT and those who received KTA. However, a meaningful difference was observed when the LTKT group was divided into the SLKT and KALT groups (P=0.047). The SLKT group had no rejection, while the KALT group showed no significant deviation compared to the KTA group (P=0.28) (Figure 4). These findings suggest that after adjusting for confounding factors through PSM, the SLKT group experienced markedly fewer rejection episodes than the KTA group.
Discussion
Our study revealed that the SLKT group exhibited no rejection during the observational period. Additionally, in the PSM analysis, the SLKT group showed a significant difference in long-term rejection-free GS compared to the KTA group. In contrast, there was no significant difference between the KALT and the KTA groups in terms of rejection-free GS. These findings imply that the immune-protective effect is more pronounced when the liver and kidney are transplanted at the same time, particularly when they are obtained from the same donor.
Our findings are consistent with previous research investigating kidney transplant outcomes in SLKT patients with similar immunological risk profiles [4,9,15,19]. These prior studies reported long-term rejection rates ranging from 4% to 10% in SLKT recipients. They also showed that the SLKT group had a considerably lower incidence of kidney graft rejection than the KTA group. In our study, the SLKT group comprised only 11 patients; 1 patient was eliminated from further follow-up due to early graft failure. Thus, 10 people served as the true sample size for studying rejection incidents in the SLKT group. If our study had a larger sample size, we would have observed rejection episodes occurring at a frequency similar to previous studies. Compared to the KTA group, the KALT group’s rejection rate did not differ significantly. Notably, few studies have addressed the results of KALT patients. Simpson et al showed that the combined liver-kidney transplantation group exhibits higher rejection-free renal GS rates at both 1 and 3 years (86% and 79%, respectively), as compared to the KALT group (75% and 61%, respectively), highlighting the potential immune-protective benefits of combined liver-kidney transplantation [9]. According to our research, the immune-protective effect against rejection might not be as strong in the KALT group, which received the liver and kidney grafts from separate donors, as in the SLKT group. Additionally, in an intra-operative kinetics study of donor-specific antibody (DSA) in SLKT patients, it was observed that DSA levels significantly decreased after liver graft perfusion, while non-DSA levels remained unchanged [20]. These findings suggest that the immune-protective effect of the liver grafts in decreasing DSA is antigen-specific.
One plausible speculation to account for the observed immune-protective effects associated with the liver allograft in our study is that the liver allograft may have contributed to the clearance or neutralization of circulating HLA antibodies [12,20,21]. The reduction in DSA was observed to start immediately following liver graft reperfusion [20]. This decrease in DSA persists for 2–3 days after LT, demonstrating comparable renal transplantation outcomes between XM-positive and XM-negative patients after LT [21]. Observational studies have indicated that SLKT leads to a decrease in DSA and subsequently results in a lower incidence of rejection episodes [4,12,15,21]. Numerous studies have consistently reported a significant decrease in DSA, particularly in HLA class I DSA, whereas HLA class II DSA displays a decreasing trend without reaching statistical significance [12,20,21]. A plausible hypothesis is that the expression of class I and class II HLA antigens in the liver parenchyma and vasculature differs from that in the renal allograft. Liver allografts primarily express MHC class I on hepatocytes, while the expression of class II molecules is less prominent [12,22]. Animal experiments also showed that non-parenchymal hepatic cells in liver grafts could effectively absorb lymphocytotoxic alloantibodies and complement, highlighting the immunological role of liver grafts in antibody removal [16].
Several hypotheses have been proposed to elucidate the mechanisms underlying the immune-protective effect of liver grafts. First, the liver’s distinct vast sinusoidal endothelial surface enables the absorption of circulating antibodies, leading to their clearance from the circulation [16,23]. In a study conducted in rat models, evidence was presented to support the rapid clearance of DSA from the circulation following LT within approximately 30 min [16]. Furthermore, with the aid of its resident phagocytic Kupffer cells, the liver secretes soluble HLA molecules with the capacity to bind and neutralize corresponding antibodies. Notably, especially in the initial post-transplant period, it has been found that the majority of circulating soluble HLA antigen molecules have the donor’s phenotype [24,25]. Additionally, the immune-protective effect of the liver can be attributed to its remarkable regenerative capacity, allowing it to recover and maintain its structure and function despite immune-mediated hepatocellular injury [25]. In the context of SLKT in comparison to KTA, it has been observed that SLKT patients demonstrate reduced occurrences of alloreactive CD4+, CD8+, effector memory, and interferon-γ producing T cells. This implies that SLKT is linked to the upregulation of gene transcription related to anti-inflammatory processes and the inhibition of immune cell subsets that cause damage [26]. Given the immune-protective mechanisms exhibited by the liver, it is reasonable to suggest that the immunotolerance effect could potentially be relevant in the context of acute cellular rejection, as well as acute and chronic antibody-mediated rejection. In the present study, an assessment was conducted on each individual instance of rejection. Nonetheless, due to the limited number of participants in the LTKT group and the overall infrequency of rejection incidents, it was difficult to conduct a full comparison between each distinct form of rejection observed un the LTKT group and the KTA group.
The surgical logistics of combined liver-kidney transplantation requires careful consideration of the timing of kidney transplantation, given the challenges presented by the simultaneous transplantation of both liver and kidney allografts. Maintaining a low central venous pressure and achieving a balanced fluid status to reduce graft congestion are necessary for maximizing liver allograft function. On the other hand, when low central venous and systolic pressures are present or vasopressors are required to keep blood pressure stable, the kidney allograft performs poorly [27]. Patients undergoing liver transplantation are typically in a compromised physiological state, with significant coagulopathy and hemodynamic instability, which can negatively impact the newly implanted kidney allograft. Moreover, patients with hyperbilirubinemia may experience bilirubin crystallization in the transplanted kidney’s tubules, which increases the risk of acute kidney injury and renal dysfunction [28]. Although surgical complications in the LTKT group did not directly result in mortality in our study, they did contribute to longer ICU stays, requiring more transfusions and vasopressor support. This illustrates that the surgical burden may be greater in the LTKT group, highlighting an increased risk of bleeding and demonstrating the potential challenges and complexities associated with these procedures. In fact, within our study population, 1 patient who underwent SLKT did not recover from DGF. To minimize surgical complications in SLKT, various approaches have been introduced, and the “Indiana approach” serves as a notable example [27]. This approach involves postponing the kidney graft implantation for 2–3 days after LT, during which time the kidney allograft is stored on a hypothermic pulsatile perfusion machine. Delaying KT provides several benefits, such as stabilizing the patien’s hemodynamic condition and coagulopathy, reducing blood loss during the KT by decompressing varices, and minimizing the risk of pressor-related DGF by weaning the patient off vasopressors before KT. Furthermore, delaying the kidney implantation also allows for the clearance of post-liver reperfusion debris and bilirubin from the circulation [29]. Machine perfusion for kidney transplantation has not been implemented in South Korea, primarily because the country’s relatively small size and efficient transportation network generally allow for cold ischemic times to be within 5–6 h, rarely exceeding 12 h. In our study cohort as well, the average cold ischemic time CIT was 305±123.9 (range: 30–995) min. Nonetheless, we anticipate that machine perfusion could become a valuable resource in multi-organ transplants such as SLKT, particularly in cases with a considerable risk of severe DGF, or in assessing the suitability of organs that might otherwise be discarded [30].
Our research has several limitations. First, it was a single-center, retrospective study, potentially constraining the applicability of the results. Moreover, notable disparities were observed in the sample sizes of the patient cohorts under investigation. To overcome the size disparities between the LTKT and KTA groups, we conducted a PSM analysis. Second, our research did not include immunological markers, such as DSA trends or protocol biopsy outcomes. Therefore, immunological effects that have not yet manifested clinically may have received insufficient analysis. However, given that the mean duration of follow-up for the included patient cohort was 148±6.9 months, we believe it was sufficient for evaluating clinical outcomes. Third, our study enrolled patients over a long time span, January 2005 to December 2020. Consequently, patients in the earlier years of the study period may have received a different perioperative management or immunosuppressive protocol than those in more recent years. Multivariate analysis was utilized to compensate for these differences. Lastly, there was the possibility that the clinician’s choice of induction agent could influence the study outcomes. To address this concern, we used a comprehensive multivariate analysis to minimize any potential influence.
Conclusions
In conclusion, our study demonstrated that SLKT is associated with a reduced incidence of rejection and improved long-term graft survival without rejection compared to KTA. However, there was no significant difference between the KALT and KTA groups in terms of rejection-free GS. These results demonstrate the immune-protective effect of concomitant liver and kidney transplantation, particularly when the organs come from the same donor. The increased rate of rejection seen in the KALT group compared to the SLKT group further strengthens this conclusion. Further research is needed to elucidate the underlying mechanisms of the immune-protective effect of liver grafts and to assess long-term outcomes in larger cohorts of SLKT recipients. Additionally, tailored patient selection for SLKT and optimization of pre- and postoperative management strategies are necessary to further enhance graft survival in patients with end-stage liver disease and chronic kidney disease.
Figures
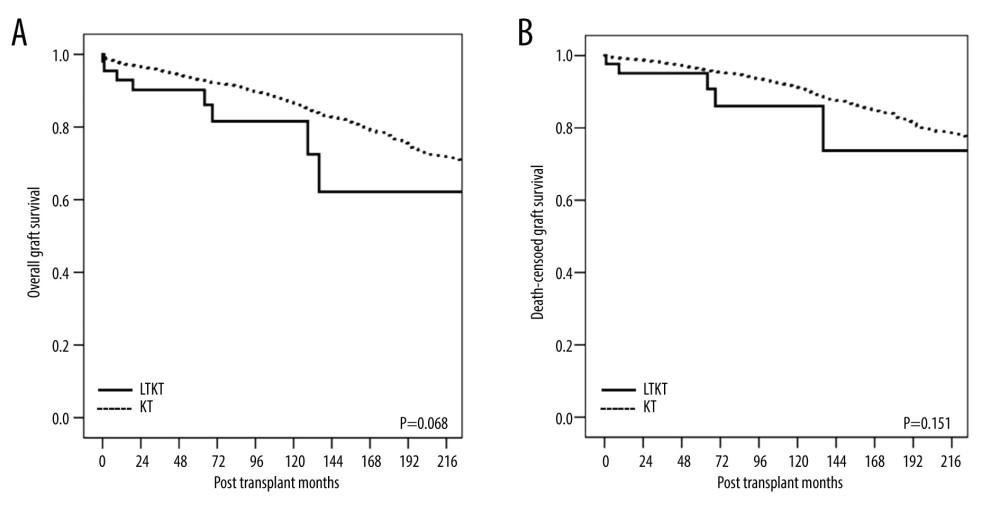 Figure 1. Kaplan-Meier curves of (A) overall graft survival and (B) death-censored graft survival for the kidney allograft. LTKT – liver-kidney transplantation; KT – kidney transplantation.
Figure 1. Kaplan-Meier curves of (A) overall graft survival and (B) death-censored graft survival for the kidney allograft. LTKT – liver-kidney transplantation; KT – kidney transplantation. 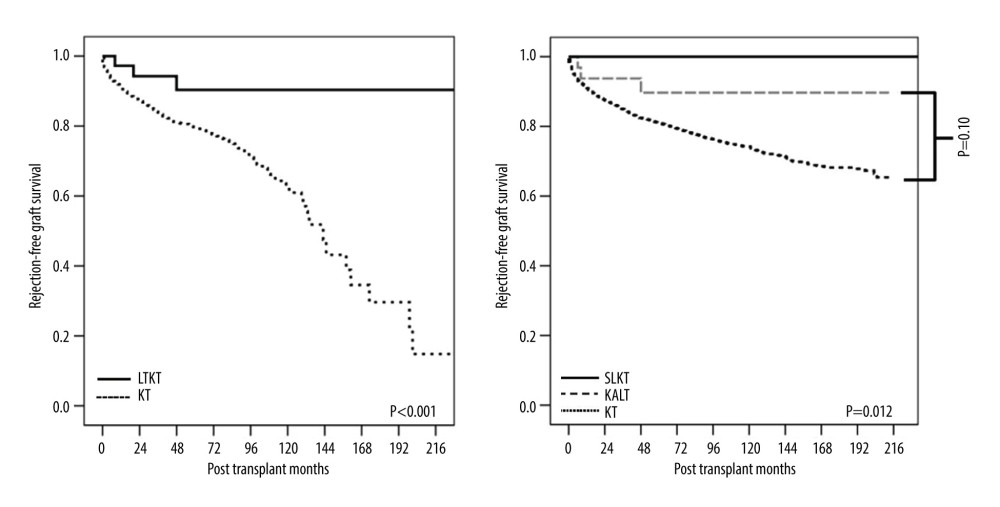 Figure 2. Kaplan-Meier curves of rejection-free survival of the kidney allograft. LTKT – liver-kidney transplantation; KT – kidney transplantation; SLKT – simultaneous liver-kidney transplantation; KALT – kidney transplantation after liver transplantation.
Figure 2. Kaplan-Meier curves of rejection-free survival of the kidney allograft. LTKT – liver-kidney transplantation; KT – kidney transplantation; SLKT – simultaneous liver-kidney transplantation; KALT – kidney transplantation after liver transplantation. 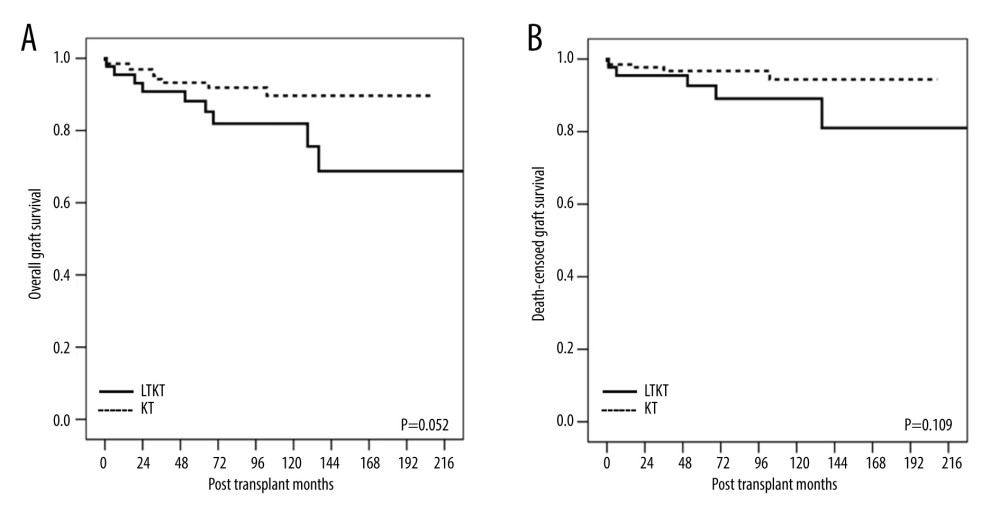 Figure 3. Kaplan-Meier curves of (A) overall graft survival and (B) death-censored graft survival for the kidney allograft in the propensity matching analysis. LTKT – liver-kidney transplantation; KT – kidney transplantation.
Figure 3. Kaplan-Meier curves of (A) overall graft survival and (B) death-censored graft survival for the kidney allograft in the propensity matching analysis. LTKT – liver-kidney transplantation; KT – kidney transplantation. 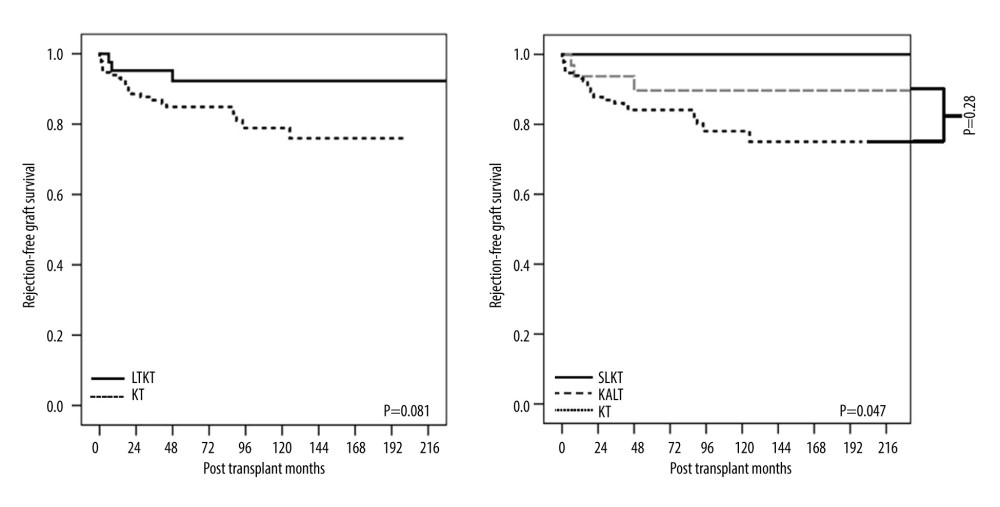 Figure 4. Kaplan-Meier curve of rejection-free graft survival for the kidney allograft in the propensity matching analysis. LTKT – liver-kidney transplantation; KT – kidney transplantation; SLKT – simultaneous liver-kidney transplantation; KALT – kidney transplantation after liver transplantation.
Figure 4. Kaplan-Meier curve of rejection-free graft survival for the kidney allograft in the propensity matching analysis. LTKT – liver-kidney transplantation; KT – kidney transplantation; SLKT – simultaneous liver-kidney transplantation; KALT – kidney transplantation after liver transplantation. Tables
Table 1. Baseline and clinical characteristics of the study patients.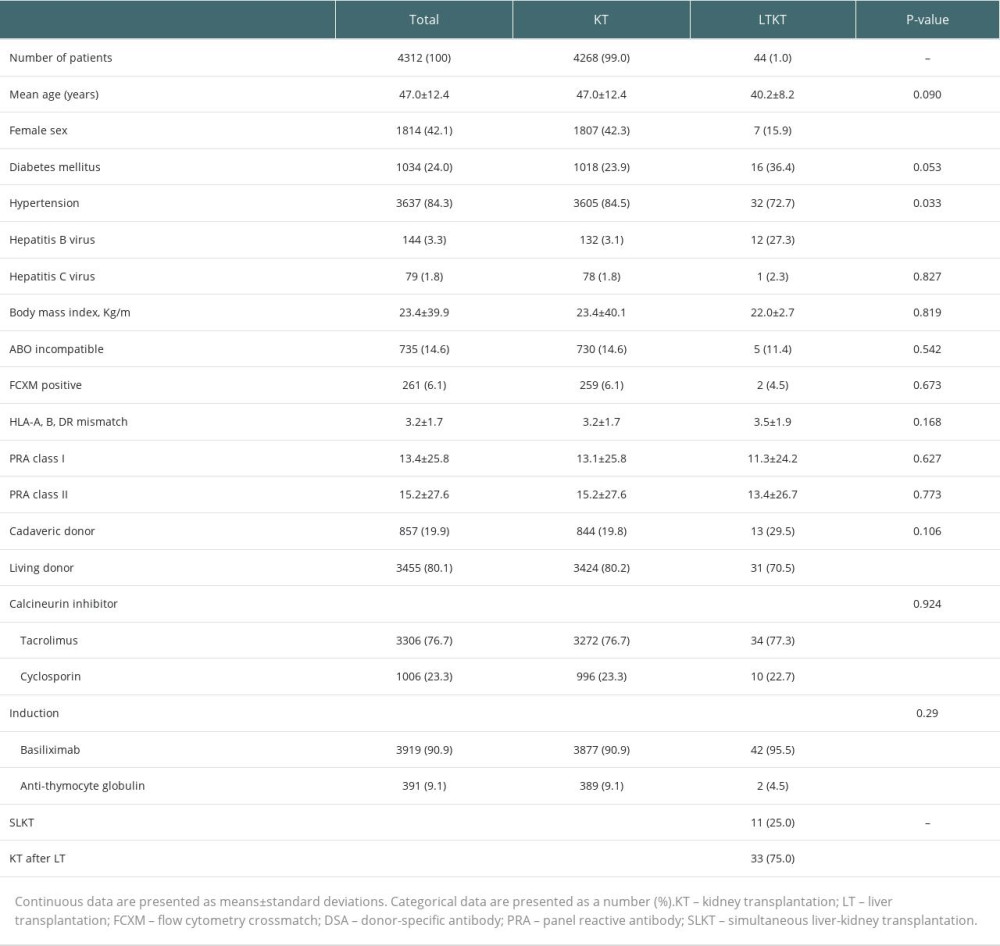 Table 2. Postoperative clinical courses of patients with SLKT.
Table 2. Postoperative clinical courses of patients with SLKT.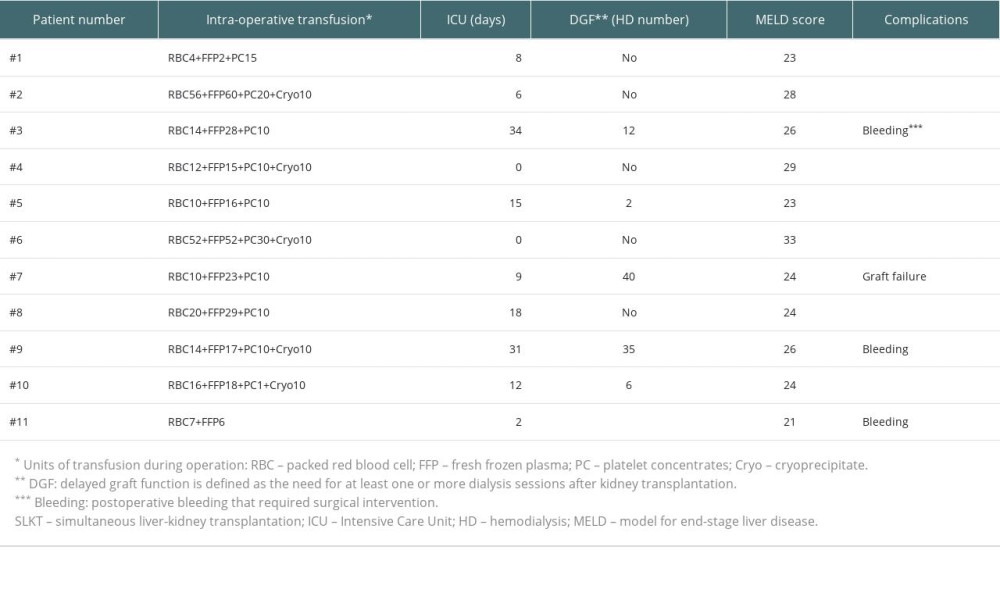 Table 3. Risk factors associated with acute rejection after transplantation.
Table 3. Risk factors associated with acute rejection after transplantation.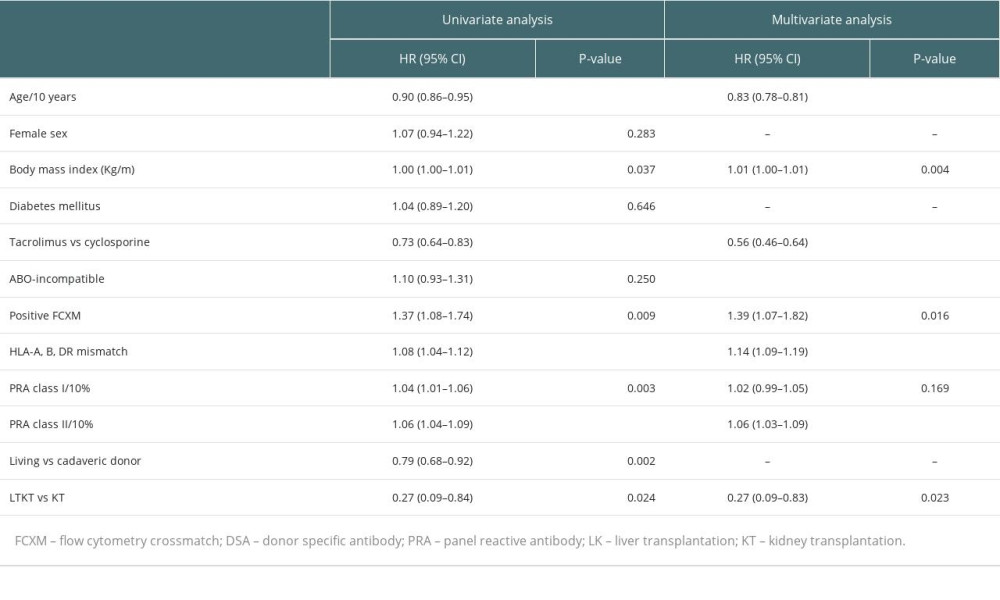 Table 4. Baseline and clinical characteristics of propensity score-matched groups.
Table 4. Baseline and clinical characteristics of propensity score-matched groups.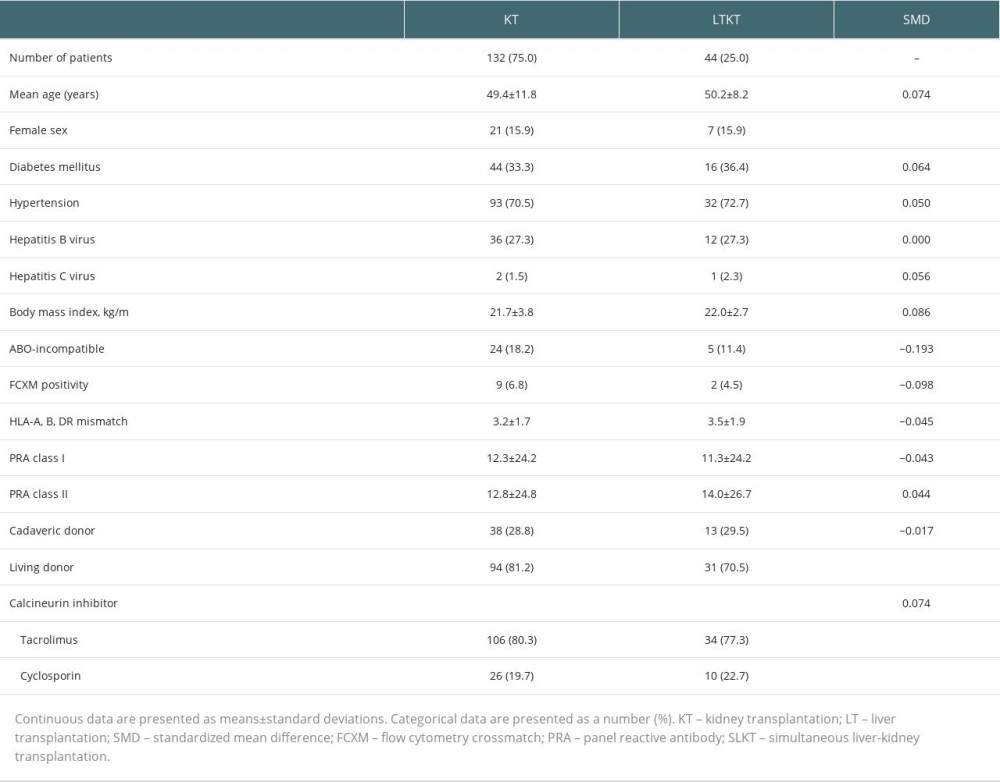 Table 5. Effect of LTKT on rejection-free graft survival of kidney transplantation after propensity score matching.
Table 5. Effect of LTKT on rejection-free graft survival of kidney transplantation after propensity score matching.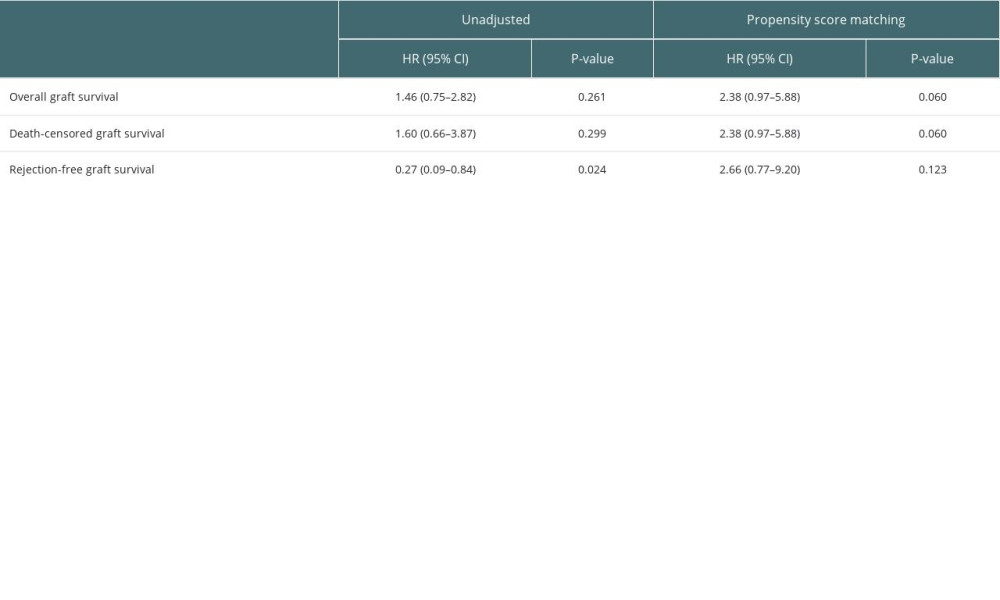 Supplementary Table 1. Comparative analysis of rejection types and incidences.
Supplementary Table 1. Comparative analysis of rejection types and incidences.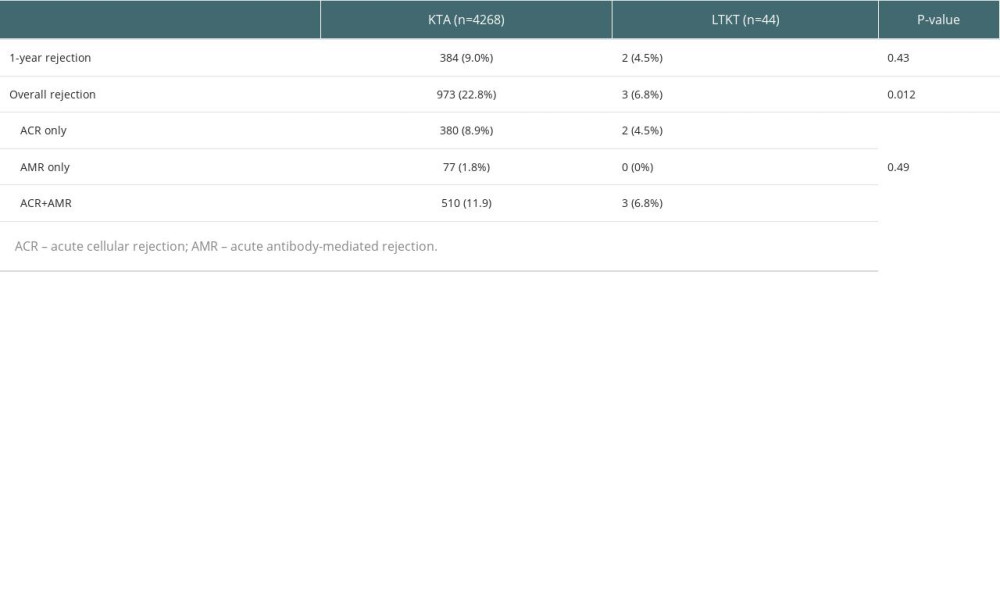
References
1. Brown RS, Lombardero M, Lake JR, Outcome of patients with renal insufficiency undergoing liver or liver-kidney transplantation: Transplantation, 1996; 62; 1788-93
2. Ginès P, Schrier RW, Renal failure in cirrhosis: N Engl J Med, 2009; 361; 1279-90
3. Thongprayoon C, Kaewput W, Thamcharoen N, Incidence and impact of acute kidney injury after liver transplantation: A meta-analysis: J Clin Med, 2019; 8(3); 372
4. Creput C, Durrbach A, Samuel D, Incidence of renal and liver rejection and patient survival rate following combined liver and kidney transplantation: Am J Transplant, 2003; 3; 348-56
5. Singal AK, Salameh H, Kuo YF, Wiesner RH, Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology: Transplantation, 2014; 98; 216-21
6. Pillebout E, Nochy D, Hill G, Renal histopathological lesions after orthotopic liver transplantation (OLT): Am J Transplant, 2005; 5; 1120-29
7. Miles CD, Westphal S, Liapakis A, Formica R, Simultaneous liver-kidney transplantation: impact on liver transplant patients and the kidney transplant waiting list: Curr Transplant Rep, 2018; 5; 1-6
8. Fong TL, Bunnapradist S, Jordan SC, Analysis of the United Network for Organ Sharing database comparing renal allografts and patient survival in combined liver-kidney transplantation with the contralateral allografts in kidney alone or kidney-pancreas transplantation: Transplantation, 2003; 76; 348-53
9. Simpson N, Cho YW, Cicciarelli JC, Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: Analysis of UNOS Database: Transplantation, 2006; 82; 1298-303
10. Hanish SI, Samaniego M, Mezrich JD, Outcomes of simultaneous liver/kidney transplants are equivalent to kidney transplant alone: A preliminary report: Transplantation, 2010; 90; 52-60
11. Leca N, Warner P, Bakthavatsalam R, Outcomes of simultaneous liver and kidney transplantation in relation to a high level of preformed donor-specific antibodies: Transplantation, 2013; 96; 914-18
12. Dar W, Agarwal A, Watkins C, Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants: Am J Transplant, 2011; 11; 841-47
13. Flye MW, Duffy BF, Phelan DL, Protective effects of liver transplantation on a simultaneously transplanted kidney in a highly sensitized patient: Transplantation, 1990; 50; 1051-54
14. Saidman SL, Duquesnoy RJ, Demetris AJ, Combined liver-kidney transplantation and the effect of preformed lymphocytotoxic antibodies: Transpl Immunol, 1994; 2; 61-67
15. Das A, Rocque B, Remulla D, Examining the role for donor-specific antibody testing in simultaneous liver-kidney transplantation: A single-center analysis of outcomes: Transplantation, 2023; 107; 1115-23
16. Gugenheim J, Amorosa L, Gigou M, Specific absorption of lymphocytotoxic alloantibodies by the liver in inbred rats: Transplantation, 1990; 50; 309-13
17. Morrissey PE, Gordon F, Shaffer D, Combined liver-kidney transplantation in patients with cirrhosis and renal failure: Effect of a positive cross-match and benefits of combined transplantation: Liver Transpl Surg, 1998; 4; 363-69
18. Haas M, Sis B, Racusen LC, Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions: Am J Transplant, 2014; 14; 272-83
19. Taner T, Heimbach JK, Rosen CB, Decreased chronic cellular and antibody-mediated injury in the kidney following simultaneous liver-kidney transplantation: Kidney Int, 2016; 89; 909-17
20. Kueht M, Jindra P, Stevenson HL, Intra-operative kinetics of anti-HLA antibody in simultaneous liver-kidney transplantation: Mol Genet Metab Rep, 2021; 26; 100705
21. Goggins WC, Ekser B, Rokop Z, Combined liver-kidney transplantation with positive crossmatch: Role of delayed kidney transplantation: Surgery, 2021; 170; 1240-47
22. Rouger P, Gugenheim J, Gane P, Distribution of the MHC antigens after liver transplantation: Relationship with biochemical and histological parameters: Clin Exp Immunol, 1990; 80; 404-8
23. Das A, Taner T, Kim J, Emamaullee J, Crossmatch, donor-specific antibody testing, and immunosuppression in simultaneous liver and kidney transplantation: A review: Transplantation, 2021; 105; e285-e91
24. Mathew JM, Shenoy S, Phelan D, Biochemical and immunological evaluation of donor-specific soluble HLA in the circulation of liver transplant recipients: Transplantation, 1996; 62; 217-23
25. Taner T, Stegall MD, Heimbach JK, Antibody-mediated rejection in liver transplantation: Current controversies and future directions: Liver Transpl, 2014; 20; 514-27
26. Taner T, Gustafson MP, Hansen MJ, Donor-specific hypo-responsiveness occurs in simultaneous liver-kidney transplant recipients after the first year: Kidney Int, 2018; 93; 1465-74
27. Ekser B, Mangus RS, Fridell W, A novel approach in combined liver and kidney transplantation with long-term outcomes: Ann Surg, 2017; 265; 1000-8
28. Ekser B, Contreras AG, Andraus W, Taner T, Current status of combined liver-kidney transplantation: Int J Surg, 2020; 82s; 149-54
29. Ekser B, Chen AM, Kubal CA, Delayed kidney transplantation after 83 hours of cold ischemia time in combined liver-kidney transplant: Transplantation, 2019; 103; e382-e83
30. Kataria A, Magoon S, Makkar B, Gundroo A, Machine perfusion in kidney transplantation: Curr Opin Organ Transplant, 2019; 24; 378-84
Figures
 Figure 1. Kaplan-Meier curves of (A) overall graft survival and (B) death-censored graft survival for the kidney allograft. LTKT – liver-kidney transplantation; KT – kidney transplantation.
Figure 1. Kaplan-Meier curves of (A) overall graft survival and (B) death-censored graft survival for the kidney allograft. LTKT – liver-kidney transplantation; KT – kidney transplantation. Figure 2. Kaplan-Meier curves of rejection-free survival of the kidney allograft. LTKT – liver-kidney transplantation; KT – kidney transplantation; SLKT – simultaneous liver-kidney transplantation; KALT – kidney transplantation after liver transplantation.
Figure 2. Kaplan-Meier curves of rejection-free survival of the kidney allograft. LTKT – liver-kidney transplantation; KT – kidney transplantation; SLKT – simultaneous liver-kidney transplantation; KALT – kidney transplantation after liver transplantation. Figure 3. Kaplan-Meier curves of (A) overall graft survival and (B) death-censored graft survival for the kidney allograft in the propensity matching analysis. LTKT – liver-kidney transplantation; KT – kidney transplantation.
Figure 3. Kaplan-Meier curves of (A) overall graft survival and (B) death-censored graft survival for the kidney allograft in the propensity matching analysis. LTKT – liver-kidney transplantation; KT – kidney transplantation. Figure 4. Kaplan-Meier curve of rejection-free graft survival for the kidney allograft in the propensity matching analysis. LTKT – liver-kidney transplantation; KT – kidney transplantation; SLKT – simultaneous liver-kidney transplantation; KALT – kidney transplantation after liver transplantation.
Figure 4. Kaplan-Meier curve of rejection-free graft survival for the kidney allograft in the propensity matching analysis. LTKT – liver-kidney transplantation; KT – kidney transplantation; SLKT – simultaneous liver-kidney transplantation; KALT – kidney transplantation after liver transplantation. Tables
 Table 1. Baseline and clinical characteristics of the study patients.
Table 1. Baseline and clinical characteristics of the study patients. Table 2. Postoperative clinical courses of patients with SLKT.
Table 2. Postoperative clinical courses of patients with SLKT. Table 3. Risk factors associated with acute rejection after transplantation.
Table 3. Risk factors associated with acute rejection after transplantation. Table 4. Baseline and clinical characteristics of propensity score-matched groups.
Table 4. Baseline and clinical characteristics of propensity score-matched groups. Table 5. Effect of LTKT on rejection-free graft survival of kidney transplantation after propensity score matching.
Table 5. Effect of LTKT on rejection-free graft survival of kidney transplantation after propensity score matching. Table 1. Baseline and clinical characteristics of the study patients.
Table 1. Baseline and clinical characteristics of the study patients. Table 2. Postoperative clinical courses of patients with SLKT.
Table 2. Postoperative clinical courses of patients with SLKT. Table 3. Risk factors associated with acute rejection after transplantation.
Table 3. Risk factors associated with acute rejection after transplantation. Table 4. Baseline and clinical characteristics of propensity score-matched groups.
Table 4. Baseline and clinical characteristics of propensity score-matched groups. Table 5. Effect of LTKT on rejection-free graft survival of kidney transplantation after propensity score matching.
Table 5. Effect of LTKT on rejection-free graft survival of kidney transplantation after propensity score matching. Supplementary Table 1. Comparative analysis of rejection types and incidences.
Supplementary Table 1. Comparative analysis of rejection types and incidences. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








