21 May 2024: Original Paper
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014–2020 Korean Organ Transplantation Registry Data Analysis
Hoonsung ParkDOI: 10.12659/AOT.943588
Ann Transplant 2024; 29:e943588
Abstract
BACKGROUND: According to the current guidelines for liver transplantation (LT) of brain-dead donors with hepatitis B or C virus (HBV or HCV) in Korea, grafts from hepatitis B surface antigen (HBsAg)(+) or HCV antibody (anti-HCV)(+) donors must be transplanted only to HBsAg(+) or anti-HCV(+) recipients, respectively. We aimed to determine the current status and outcomes of brain-dead donor LT with HBV or HCV in Korea.
MATERIAL AND METHODS: This retrospective observational study included all LTs from brain-dead donors in the Korean Organ Transplantation Registry between April 2014 and December 2020. According to donor hepatitis status, 24 HBV(+), 1 HCV(+), and 1010 HBV(-)/HCV(-) donors were included.
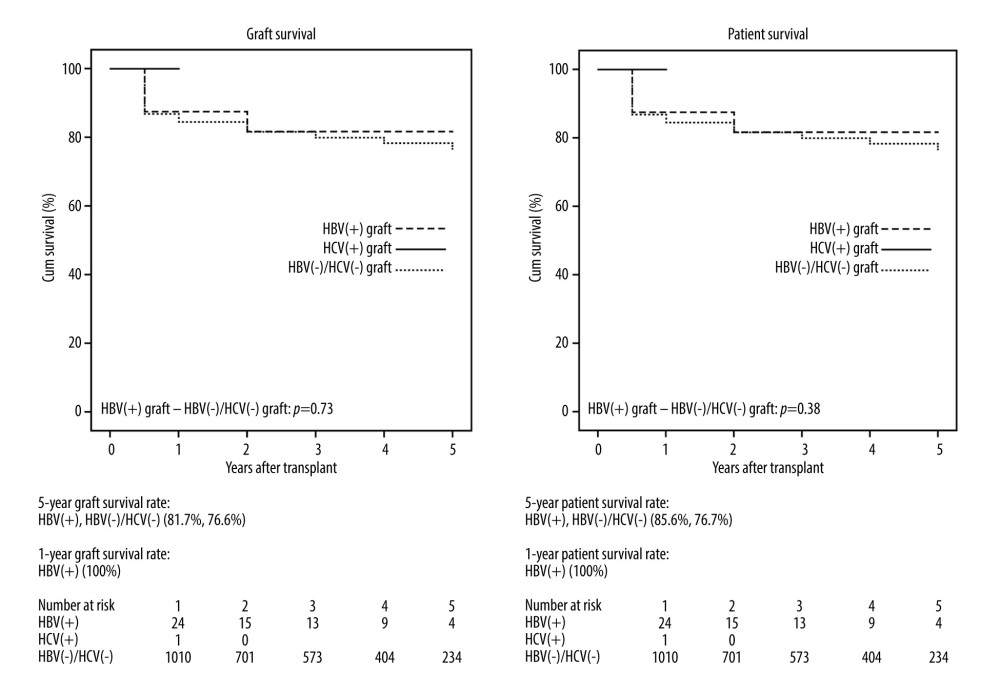
RESULTS: Baseline/final model for end-stage liver disease score (MELD) for HBV(+), HCV(+), and HBV(-)/HCV(-) were 22.4±9.3/27.8±7.8, 16/11, and 33.0±15.4/35.5±7.1, respectively. MELD score of HBV (+) were lower than those of HBV(-)/HCV(-) (P<0.01). Five-year graft and patient survival rates of HBV(+) and HBV(-)/HCV(-) recipients were 81.7%/85.6%, and 76.6%/76.7%, respectively (P=0.73 and P=0.038). One-year graft and patient survival rates of HCV (+) graft recipients were both 100%.
CONCLUSIONS: No differences in graft and patient survival rates between HBV(+) and HBV(-)/HCV(-) groups were observed. Although accumulating the results of transplants from HBV (+) or HCV(+) grafts to HBV(-) or HCV(-) recipients is not possible owing to domestic regulations, Korea should conditionally permit transplantations from HBV(+) or HCV(+) grafts to HBV(-) or HCV(-) recipients by considering the risks and benefits based on foreign studies. Thereafter, we can accumulate the data from Korea and analyze the outcomes.
Keywords: Brain Death, Hepatitis B, Hepatitis C, Liver Transplantation, Tissue Donors
Introduction
As of 2020, 6102 people were waiting for liver transplants in Korea, which accounts for 14% of all organ transplant waiting lists and has increased annually from 4422 in 2014 [1]. To resolve donor–recipient disparity due to the organ shortage, the concept of expanded criteria donors was introduced, and donors with hepatitis B and C virus (HBV and HCV) were included [2]. Under the current Center for Korean Network for Organ Sharing (KONOS) guideline, in cases of liver transplantation (LT) of a deceased brain-dead donor with HBV or HCV in Korea, grafts from hepatitis B surface antigen (HBsAg)(+) donors must be transplanted only to HBsAg(+) recipients, and the grafts from HCV antibody (anti-HCV)(+) donors must also be transplanted to anti-HCV(+) recipients [3]. Previous studies have shown that the transplantation of an antibody to hepatitis B core antigen (anti-HBc)(+) graft is safe, and peri-transplantation antiviral prophylaxis helps to obtain similar post-transplant outcomes [4–7]. Although the transplantation of HBsAg(+) grafts remains controversial, many studies have reported positive outcomes of transplantation using HBsAg(+) liver grafts [8–11]. We aimed to determine the current status and outcomes of brain-dead donor LT in patients with HBV or HCV in Korea.
Material and Methods
This retrospective observational study used data from the Korean Organ Transplantation Registry (KOTRY) database between April 2014 and December 2020. Data included all LTs from brain-dead donors in the KOTRY database, which were divided into 3 groups according to their hepatitis status. A total of 1035 LTs were performed, including 24 from HBV(+) donors, 1 from HCV(+) donors, and 1010 from HBV(−)/HCV(−) donors. No patients were excluded owing to a lack of data or other reasons. All continuous data are presented as medians, standard deviations, and ranges. Categorical data are presented as numbers. All statistical analyses were performed using SPSS Statistics for Windows (version 26.0; IBM Corp., Armonk, NY). The chi-square, Fisher’s exact, and
Results
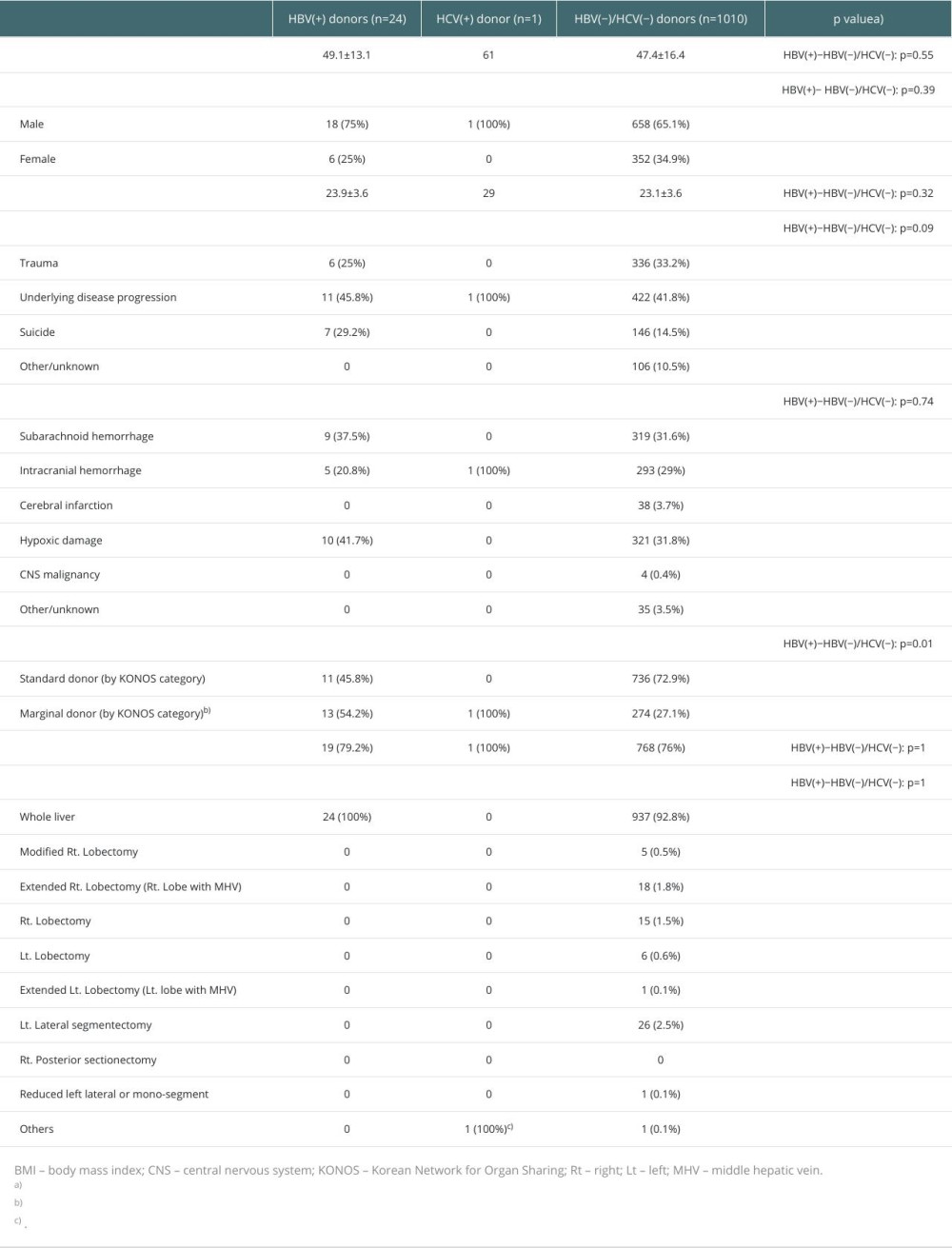
Table 1 presents the characteristics of the donors in the 3 groups. Because there was only 1 HCV(+) donor, statistical analysis was only performed between HBV(+) and HBV(−)/HCV(−) donors. No statistically significant differences were noted between the HBV(+) and HBV(−)/HCV(−) donor groups, except for the donor type. The causes of brain death in HBV(+) donors were disease progression (n=11, 45.8%), suicide (n=7, 29.2%), and trauma (n=6, 25%). In HCV(+) donors, underlying disease progression was the single cause of brain death (n=1, 100%). Among the HBV(−)/HCV(−) donors, underlying disease progression (n=422, 41.8%), trauma (n=336, 33.2%), suicide (n=146, 14.5%), and other or unknown causes (n=106, 10.5%) were the causes of brain death. The mechanisms of brain death in the HBV(+) donors included hypoxic damage (n=10, 41.7%), subarachnoid hemorrhage (n=9, 37.5%), and intracranial hemorrhage (n=5, 20.8%). Intracranial hemorrhage was the single mechanism of brain death in HCV(+) donors. In the HBV(−)/HCV(−) donors, hypoxic damage (n=321, 31.8%), subarachnoid hemorrhage (n=319, 31.6%), intracranial hemorrhage (n=293, 29%), cerebral infarction (3.7%), others/unknown (n=35, 3.5%), and central nervous system malignancy (n=4, 0.4%) were the mechanisms of brain death. Donor types in the HBV(+) group were 11 (45.8%) standard and 13 (54.2%) marginal donors. In the HCV(+) donors, only 1 marginal donor was observed. In the HBV(−)/HCV(−) donors, 736 (7.9%) standard and 274 (27.1%) marginal donors were observed. The ratio of standard donors was significantly higher in the HBV(−)/HCV(−) donors than that in the HBV(+) donors (72.9% vs 45.8%, HBV(+) and HBV(−)/HCV(−), p=0.01).
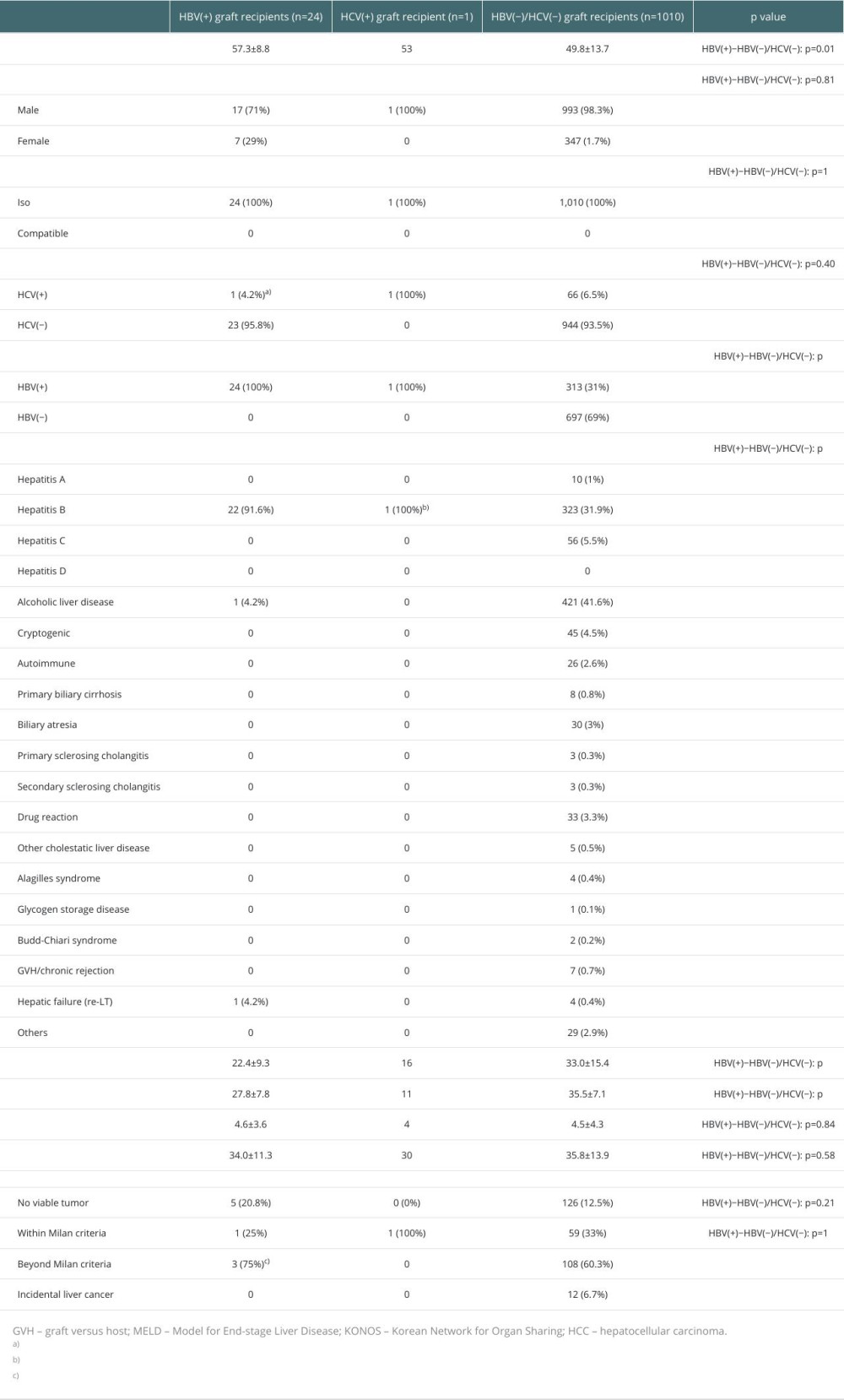
The characteristics of the recipients in the 3 groups are presented in Table 2. The median age of the HBV(+), HCV(+), and HBV(−)/HCV(−) donors were 57.3±8.8, 53, and 49.8±13.7, respectively (HBV[+]−HBV[−]/HCV[−], p=0.01). The median age of the HBV(−)/HCV(−) graft recipients was lower than that of the HBV(+) graft recipients. The ratio of males to females was higher in the HBV(+) graft recipients than that in the HBV(−)/HCV(−) graft recipients (male/female; 71%/29% and 98.3%/1.7%, p=0.81). All LTs in the 3 groups were ABO-ISO. Among the HBV(+) graft recipients, 24 were HBV(+) and 1 was HCV(+); the latter was both HBV(+) and HCV(+). Only 1 HCV(+) graft recipient was HBV(+) and HCV(+). In the HBV(−)/HCV(−) grafts, 66 (6.5%) and 313 (31%) were HCV(+) and HBV(+) recipients, respectively. Among HBV(+) graft recipients with primary liver diseases, 22 (91.6%) had HBV, 1 (4.2%) had alcoholic liver disease, and 1 (4.2%) had hepatic failure (re-LT). For the HCV(+) graft recipient, HBV was the primary liver disease. Among the HBV(−)/HCV(−) graft recipients, 421 (41.6%) had alcoholic liver disease; 323 (31.9%) had HBV, 45 (5.5%) had HCV; 45 (4.5%) were cryptogenic; 30 (3%) had biliary atresia, 33 (3.3%) had drug reactions, 26 (2.6%) had autoimmune diseases, 8 (0.8%) had primary biliary cirrhosis, 7 (0.7%) had graft-versus-host disease/chronic rejection, and 29 (2.9%) had other diseases. The ratio of HBV was higher in the HBV(+) graft recipient group than in the HBV(−)/HCV(−) graft recipient group (HBV[+]-HBV[−]/HCV[−],
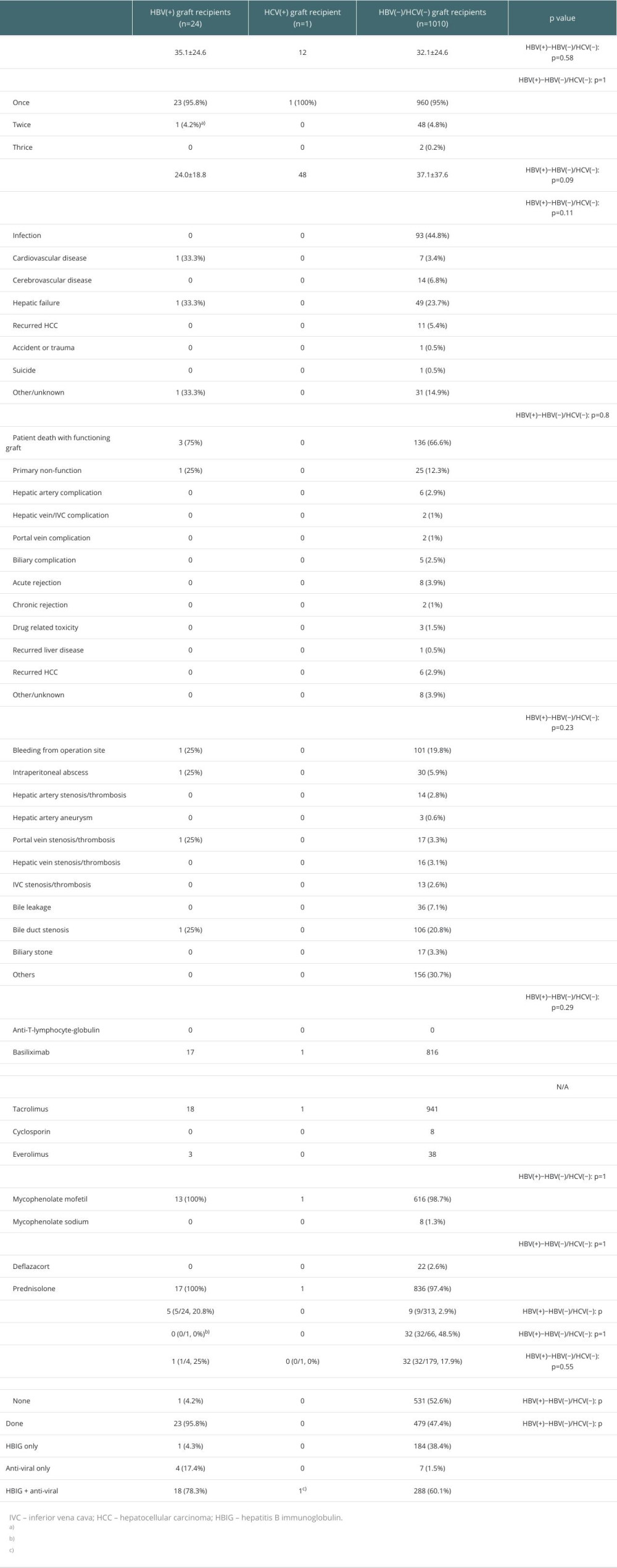
The outcomes of LTs are presented in Table 3. No statistically significant differences were observed in the follow-up period, number of LTs, postoperative hospital days, cause of death, cause of graft loss, complications, immunosuppressant use for induction therapy, HCV recurrence, or HCC recurrence between the HBV(+) and HBV(−)/HCV(−) graft recipients. The HBV recurrence rate was significantly higher in the HBV(+) graft recipients than in the HBV(−)/HCV(−) graft recipients (20.8% vs 2.9%, HBV[+]−HBV[−]/HCV[−],
Graft and patient survival rates at 5 years after LT are shown in Figure 1. The 5-year graft survival rates of the HBV(+), HBV(−)/HCV(−) recipients were 81.7 and 76.6%, respectively (HBV[+]−HBV[−]/HCV[−],
Discussion
LIVER TRANSPLANT USING HBV(+) GRAFTS:
In Western countries, transplantation from anti-HBc(+) donors to anti-HBc(−) recipients is more common than transplantation from HBsAg(+) donors to HBsAg(−) recipients. In the case of transplantation from anti-HBc(+) donors to anti-HBc(−) recipients, the risk of HBV infection reactivation is very low, and the risk of de novo hepatitis is negligible, even without prophylaxis, regardless of the recipient’s HBV immune status. Additionally, its safety has been demonstrated in several studies, not only in LT but also in organ transplantation, such as the kidney, lung, and heart [12–14].
Organs that are HBsAg(+) are not routinely utilized, but are utilized in urgent cases with sufficient informed consent [15]. In Korea, transplantation must be implemented from HBsAg(+) donors to HBsAg(+) recipients, and from anti-HCV(+) donors to anti-HCV(+) recipients. The KOTRY and KONOS databases do not collect data on donor anti-HBc titers. Therefore, in this study, HBV(+) refers to HBsAg(+), and HCV(+) refers to anti-HCV(+).
In HBV-endemic areas, such as East Asia (Korea, China, and Japan), activation of the transplants using HBsAg(+) grafts can be considered. Although the completeness of surgery is important, appropriate administration of antiviral agents and HBIG through appropriate prophylactic and maintenance therapies, according to the virological status of the donor/recipient, is also important. In this study, the rate of prophylaxis treatment for HBV was higher in the HBV(+) group than that in the HBV(−)/HCV(−) group (95.8% vs 47.4%), which is thought to be due to the virological characteristics of the grafts in both groups.
The baseline and final MELD scores were lower in the HBV(+) graft recipients than in the HBV(−)/HCV(−) recipients (Table 2). This may be due to the social atmosphere, in which many people on waiting lists are reluctant to receive hepatitis (+) grafts. Therefore, hepatitis (+) grafts have lower priority, are stable, and have lower MELD scores.
LIVER TRANSPLANT USING HCV(+) GRAFTS:
In the United States, HCV(+) donors accounted for 3% of deceased cadaveric liver donor pool between 2007 and 2010. Grafts that are HCV(+) have been used for HCV-viremic recipients because of de novo HCV infection. However, with the advent of direct-acting antiviral (DAA) in 2013, the number of transplants has increased [2]. The incidence of HCV infection in the United States has been increasing because of the opioid crisis. According to the Centers for Disease Control and Prevention data, between 2004 and 2014, HCV infection increased by 4-fold among those aged 18–29 years, and 3.25-fold among those aged 30−39 years [16]. A study based on the Mid-America Transplant Services database reported that approximately 10% of HCV(+) livers in the United States were discarded because of HCV infection between 2014 and 2017 [17].
According to the 2013−2017 KONOS and Korea Organ Donation Agency databases, of a total of 87 cases of organ transplantation of brain-dead donors from HCV(+) donors, 11 were transplanted, and of the 76 cases in which the transplantation failed, 24 (27.5%) were due to recipient refusal and 24 (27.5%) were not suitable donors. This may reflect the reluctance of recipients who do not want to receive HCV(+) grafts [18].
One of the important factors for the activation of extended-criteria donors is a change in the perception of recipients and their families about the safety and benefits of grafts from extended-criteria donors.
Between 2013 and 2017, recipients’ refusal of brain-dead donors liver transplants from HBV(+) or HCV(+) donors accounted for 27% of all the donation failures in South Korea [18]. This suggests that despite being HBV(+) or HCV(+), many individuals do not wish to receive organ transplants from HBV(+) or HCV(+) donors. Donors who were infected with HIV, HBV, or HCV were categorized as “increased-risk donors” (IRD) by the US Public Health Service in 2013 [19]. In several studies, the IRD organ rejection rate ranged from 24% to 98.5%, and the IRD organ rejection rate in pediatric kidney transplantation in the United States was 98.5% [20,21]. As recipients benefit from the use of IRD organs, it is important to convince patients that the advantages of using this organ exceeds the risks. Patients and their families must be informed of the advantages and safety of organ donation from HBV(+) and HCV(+) donors.
This study has some limitations. First, the effects of selection bias due to its retrospective nature and the effects of confounding factors due to its observational nature cannot be excluded. Second, it was impossible to implement a statistical analysis in the HCV(+) group because it consisted of only 1 case. Additionally, there were fewer HBV(+) patients than HBV(−)/HCV(−) patients and the number of HBV(+) patients was very low. Therefore, the statistical power may have been decreased.
Conclusions
No differences were observed in graft and patient survivals between the HBV(+) and HBV(−)/HCV(−) groups. However, a statistical analysis was not possible in the HCV (+) group because of the small sample size. The number of transplants in the HBV(+) and HCV(+) groups was very small compared to that in the HBV(−)/HCV(−) group. However, the transplant outcomes of the HBV(+) group were comparable to that of the HBV(−)/HCV(−) group. Although accumulating the results of transplants from HBV(+) grafts to HBV(−) recipients and from HCV(+) grafts to HCV(−) recipients is not possible owing to domestic regulations, there should be conditional permission for transplantation from HBV(+) or HCV(+) grafts to HBV(−) or HCV(−) recipients in Korea by considering the risks and benefits based on foreign studies. Thereafter, we can accumulate data from Korea and analyze the outcomes. With the advent of DAA, HCV control has become possible and HBV prophylaxis is now common. Considering that the number of people waiting for a liver transplant is increasing daily, this can no longer be delayed. Therefore, we hope HBV(+) to HBV(−) and HCV(+) to HCV(−) transplantations will be implemented in Korea as soon as possible.
References
1. Korean Network for Organ Sharing (KONOS): 2015–2020 Annual data report Available from: https://www.konos.go.kr
2. Feng S, Lai JC, Expanded criteria donors: Clin Liver Dis, 2014; 18; 633-49
3. Korean Network for Organ Sharing (KONOS): 2021 Guidelines for organ transplantation management 8e Available from: http://www.konos.go.kr
4. Angelico M, Nardi A, Marianelli T, Hepatitis B-core antibody positive donors in liver transplantation and their impact on graft survival: Evidence from the Liver Match cohort study: J Hepatol, 2013; 58; 715-23
5. Wong TC, Fung JY, Cui TY, Liver transplantation using hepatitis B core positive grafts with antiviral monotherapy prophylaxis: J Hepatol, 2019; 70; 1114-22
6. Cholongitas E, Papatheodoridis GV, Burroughs AK, Liver grafts from anti-hepatitis B core positive donors: A systematic review: J Hepatol, 2010; 52; 272-79
7. Kim HY, Choi JY, Park CH, Adult living donor liver transplantation using hepatitis B core antibody-positive grafts in Korea, a hepatitis B-endemic region: Gut Liver, 2011; 5; 363-66
8. Hwang S, Lee SG, Park KM, Five-year follow-up of a hepatitis B virus-positive recipient of hepatitis B surface antigen-positive living donor liver graft: Liver Transpl, 2006; 12; 993-97
9. Ballarin R, Cucchetti A, Russo FP, Long term follow-up and outcome of liver transplantation from hepatitis B surface antigen positive donors: World J Gastroenterol, 2017; 23; 2095-105
10. Li Z, Hu Z, Xiang J, Use of hepatitis B surface antigen-positive grafts in liver transplantation: A matched analysis of the US National database: Liver Transpl, 2014; 20; 35-45
11. Wei L, Chen D, Zhang B, Long-term outcome and recurrence of hepatitis B virus following liver transplantation from hepatitis B surface antigen-positive donors in a Chinese population: J Viral Hepat, 2018; 25; 1576-81
12. Huprikar S, Danziger-Isakov L, Ahn J, Solid organ transplantation from hepatitis B virus-positive donors: Consensus guidelines for recipient management: Am J Transplant, 2015; 15; 1162-72
13. The Transplantation Society of Australia and New Zealand: Clinical guidelines for organ transplantation from deceased donor’s version 1.4 Available from: https://tsanz.com.au/storage/documents/TSANZ_Clinical_Guidelines_Version-14.pdf
14. British Transplant Society: Guidelines for Hepatitis B & Solid Organ Transplantation, 2018 Available from: https://bts.org.uk/wp-content/uploads/2018/03/BTS_HepB_Guidelines_FINAL_09.03.18.pdf
15. Levitsky J, Doucette KAST Infectious Diseases Community of Practice, Viral hepatitis in solid organ transplantation: Am J Transplant, 2013; 13; 147-68
16. Zibbell JE, Asher AK, Patel RC, Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, united states, 2004 to 2014: Am J Public Health, 2018; 108; 175-81
17. Keller J, Marklin G, Okoye O, Treatment of hepatitis C post-liver transplantation could mitigate discard rates of hepatitis C-positive deceased donor livers and expand the donor pool: J Transplant, 2021; 2021; 6612453
18. Park H, Jung ES, Lee MH, Lee JM, Organ donation from donors with hepatitis B or C in South Korea: A 2013–2017 Nationwide Data Analysis: Ann Transplant, 2021; 26; e928947
19. Ruck JM, Segev DL, Expanding deceased donor kidney transplantation: Medical risk, infectious risk, hepatitis C virus, and HIV: Curr Opin Nephrol Hypertens, 2018; 27; 445-53
20. Reese PP, Tehrani T, Lim MA, Determinants of the decision to accept a kidney from a donor at increased risk for blood-borne viral infection: Clin J Am Soc Nephrol, 2010; 5; 917-23
21. Wrenn SM, Callas PW, Kapoor T, Increased risk organ transplantation in the pediatric population: Pediatr Transplant, 2017; 21; 13041
Figures
In Press
Original article
Effect of the Organ Donation Quality System on Donation Activity of Warsaw HospitalsAnn Transplant In Press; DOI: 10.12659/AOT.943520
Original article
Clonal Hematopoiesis-Associated Gene Mutations Affect Acute Graft-Versus-Host Disease After Hematopoietic S...Ann Transplant In Press; DOI: 10.12659/AOT.943688
Original article
Impact of Recipient and Donor Body Mass Index on Survival Outcomes After Intestinal Transplantation: A Unit...Ann Transplant In Press; DOI: 10.12659/AOT.943994
Original article
Ginkgetin Pretreatment Reduces Inflammatory Response in DCD Donor Liver via JAK2/STAT3 Signaling PathwayAnn Transplant In Press; DOI: 10.12659/AOT.944153
Most Viewed Current Articles
05 Apr 2022 : Original article 12,210
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
22 Nov 2022 : Original article 8,764
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
12 Jan 2022 : Original article 8,649
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
15 Mar 2022 : Case report 6,500
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860