30 January 2024: Original Paper
Cyclosporine A Does Not Mitigate Liver Ischemia/Reperfusion Injury in an Ex Vivo Porcine Model of Donation After Circulatory Death
Joshua HeflerDOI: 10.12659/AOT.941054
Ann Transplant 2024; 29:e941054
Abstract
BACKGROUND: Ischemia/reperfusion injury (IRI) is an inherent problem in organ transplantation, owing to the obligate period of ischemia that organs must endure. Cyclosporine A (CsA), though better know as an immunosuppressant, has been shown to mitigate warm IRI in a variety of organ types, including the liver. However, there is little evidence for CsA in preventing hepatic IRI in the transplant setting.
MATERIAL AND METHODS: In the present study, we tested the effect of CsA on hepatic IRI in a large-animal ex vivo model of donation after circulatory death (DCD). Porcine donors were pre-treated with either normal saline control or 20 mg/kg of CsA. Animals were subject to either 45 or 60 minutes of warm ischemia before hepatectomy, followed by 2 or 4 hours of cold storage prior to reperfusion on an ex vivo circuit. Over the course of a 12-hour perfusion, perfusion parameters were recorded and perfusate samples and biopsies were taken at regular intervals.
RESULTS: Peak perfusate lactate dehydrogenase was significantly decreased in the lower-ischemia group treated with CsA compared to the untreated group (4220 U/L [3515-5815] vs 11 305 [10 100-11 674]; P=0.023). However, no difference was seen between controls and CsA-treated groups on other parameters in perfusate alanine or asparagine aminotransferase (P=0.912, 0.455, respectively). Correspondingly, we found no difference on midpoint histological injury score (P=0.271).
CONCLUSIONS: We found minimal evidence that CsA is protective against hepatic IRI in our DCD model.
Keywords: Cyclosporine, Liver Transplantation, Models, Animal, Reperfusion Injury, Tissue and Organ Procurement
Background
Ischemia/reperfusion injury (IRI) occurs following the return of blood flow to previously ischemic tissue [1]. Ischemia itself is characterized by metabolic derangements, including depletion of the adenosine triphosphate (ATP) reserve with concomitant increase in lactic acid. Prolonged ischemia results in cell death by mechanisms such as the loss of cellular membrane potential. Cell death is exacerbated upon the return of blood flow by the intense generation of reactive oxygen species (ROS) and the initiation of a proinflammatory cascade, including the recruitment of immune cells and activation of the complement system [2].
IRI is relevant in several clinical scenarios, in which organs are partially or wholly deprived of blood flow for a period of time. Organ transplantation is a notable example, as there is an obligate period of ischemia from when the organ is explanted from the donor to when it is implanted into the recipient. For the most part this occurs during cold storage, where the organ is kept at 4ºC to minimize its metabolic demands [3]. However, due to rising demand, organs are increasing being used following circulatory death, where there is an additional period of warm ischemia before the organs can be procured [4]. Organs donated after circulatory death (DCD) are more susceptible to IRI, which, for the liver, means higher risk of primary non-function and decreased graft survival [5].
Cyclosporine A (CsA) is one of the early immunosuppressant drugs that enabled the success of solid organ transplantation. Its immunosuppressive function is achieved through the inhibition of calcineurin, which prevents T cell activation [6]. An additional effect of CsA is its inhibition of the mitochondria permeability transition pore (mPTP) via its binding to cyclophilin d [7]. This secondary function has been explored to prevent mitochondrial swelling and dysfunction in the setting of IRI.
CsA has been demonstrated to reduce warm hepatic IRI in several preclinical models, including a porcine model of hepatic artery clamping [8–10]. However, the evidence in transplantation-related models combining warm and cold ischemia is less robust and does not include any large-animals studies [11,12] In the present study, we explore the effect of CsA in a more clinically relevant large-animal model of hepatic IRI following donation after circulatory death.
Material and Methods
STUDY DESIGN:
This study consisted of an ex vivo model of liver DCD, in which livers enduring a period of warm ischemia followed by cold ischemia were subject to whole-blood reperfusion using an ex vivo perfusion device. Four groups of 5 animals each were included in the study, testing 2 levels of ischemic injury with or without CsA (Figure 1). Group size was optimized based on the resource equation method [13]. At the lower degree of ischemic injury, livers were subject to 45 minutes of warm ischemia, followed by 2 hours of cold ischemia (at 4ºC), whereas the higher degree of injury consisted of 60 minutes of warm ischemia, followed by 4 hours of cold ischemia. A dose of either normal saline (control) or 20 mg/kg CsA (treatment) was administered prior to the onset of warm ischemia.
LIVER PROCUREMENT:
For liver procurement, pigs were sedated with 20 mg/kg of ketamine (Ketaset; Zoetis Canada Inc., Kirkland, QC, Canada) and 0.05 mg/kg of atropine (Atro-SA; Rafter 8 Products Inc., Calgary, AB, Canada). They then underwent insertion of an endotracheal tube and received inhalational anesthesia (3–4% isoflurane; Frensenius Kabi Canada, Toronto, ON, Canada). The stomach was decompressed via orogastric tube insertion and a 20 G venous cannula was used in an ear vein for fluid administration. A cut-down to the right carotid artery was performed to insert an arterial pressure monitoring line.
Either control saline or drug were infused via the ear vein. A 30-minute period was allowed for the treatment to circulate prior to proceeding with abdominal dissection. A midline laparotomy was performed extending from the xyphoid process to pubic synthesis. Peritoneum overlying the infra-renal aorta was incised and a segment of aorta was dissected and encircled with 0 silk ties (Perma Hand; Ethicon, Inc., Bridgewater, NJ, USA). Next, the gallbladder was removed from the surface of the liver using a combination of electrocautery and blunt dissection, ligating the cystic duct and artery together. The main portal vein and supraceliac aorta were then dissected in turn and encircled with a 2-0 silk for later identification. A thoracotomy was performed to facilitate blood collection via the right atrium, which was achieved by inserting a 2-staged venous canula. Exsanguination was performed and blood was collected (1.5–2 L) into a sterile container. Warm ischemic time was measured from the point at which systolic blood pressure dropped below 50 mmHg.
Following the period of warm ischemia, the supraceliac aorta was clamped and a cannula was inserted into the infra-renal aorta through which 2 L of cold University of Wisconsin (UW) solution (Belzar UW® Cold Storage Solution; Bridge to Life, Ltd., Colombia, SC, USA) was infused. Ice was then packed into the abdomen and the suprahepatic inferior vena cava (IVC) was cut for venting of the flush. After flushing, hepatectomy was performed. The liver was weighed, flushed on the back table via the portal vein with an additional 0.5 L of UW solution, and placed on ice slush in an organ retrieval bag for the duration of the cold ischemic time.
NMP PERFUSION:
Upon completion of the cold ischemic period, livers were perfused for 12 hours according to our previously established protocol [14,15]. As illustrated in Figure 2, perfusate is circulated via 2 BPX-80 Bi-Medicus centrifugal pumps (Medtronic of Canada, Ltd., Brampton, ON, Canada). One pump directs perfusate through an EOS ECMO oxygenator (Sorin Group Canada, Inc., Burnaby, BC, Canada) before entering the hepatic artery, while the second pump directs perfusate through a MYOtherm XP heat exchanger (Medtronic of Canada, Ltd., Brampton, ON, Canada) before it enters the portal vein. Perfusate drained from the liver’s IVC into a filtered CARD EVO cardiotomy reservoir (Sorin Group Canada, Inc., Burnaby, BC, Canada) before returning to the pumps. Perfusate was heated as it passed through the heat exchanger and oxygenator by CW-05G water bath (Lab Companion, Jeio Tech, Inc., Billerica, MA, USA). Transonic Clamp-on Tubing Flowsensors (6PXL and 7PXL; Transonic Systems, Inc., Ithaca, NY, USA) measured arterial and portal venous flows, while pressures were measured using TruWave pressure transducers (Edwards Life Sciences Canada Inc., Mississauga, ON, Canada). The speed of the pumps is controlled by computer at a set hepatic artery pressure and portal venous flow. Total hepatic blood flow was targeted to be approximately 800 ml/min, in accordance with known values [16]. Inflows of oxygen and carbon dioxide are adjusted to keep physiologic partial pressures of arterial oxygen (100–120 mmHg) and carbon dioxide (35–45 mmHg).
Prior to commencing perfusion, the circuit was primed with a 1: 1 ratio of whole blood to modified Krebs-Henseleit solution (glucose [5 mM], NaCl [85 mM], KCl [5 mM], CaCl2 [1 mM], MgCl2 [1 mM], NaHCO3 [25 mM], NaH2PO4 [1 mM], sodium pyruvate [5 mM], and 8% bovine serum albumin). Piperacillin/tazobactam (3.375 g; Sandoz Canada, Boucherville, QC, Canada), methylprednisolone (500 mg; Solu-Medrol, Pfizer Canada Inc., Kirkland, QC, Canada), and sodium heparin (5000 U; Fresenius Kabi Canada, Toronto, ON, Canada) were given initially as boluses. Infusions of regular insulin (2 U/h; Humulin R; Eli Lilly & Company, Toronto, ON, Canada) and sodium heparin (1000 U/h) were given during perfusion. Boluses of trisaminomethane (THAM, 3mol/L; Sigma-Aldrich Canada, Oakville, ON, Canada) and glucose (250 mg/mL; Sigma-Aldrich Canada, Oakville, ON, Canada) were given as needed to maintain perfusate pH and glucose within physiologic range (7.35–7.45 and 6–10 mmol/L, respectively).
Each liver was flushed with 2 L of normal saline prior to canulation to remove the UW solution. An 8 Fr arterial cannula (Medtronic of Canada, Ltd., Brampton, ON, Canada) and 1/4’ polycarbonate tubing connector were used to attach the hepatic artery and portal vein to the circuit, respectively. The common bile duct was cannulated with a thin piece of tubing draining to a separate reservoir. Arterial and portal venous flows were increased to target values, as the liver reached a physiologic temperature of 37°C.
PERFUSATE BIOCHEMISTRY:
Perfusate hemoglobin, pH, oxygen and carbon dioxide partial pressures, electrolytes, glucose, and lactate were measured in real time using an automated point-of-care blood gas analyzer (ABL Flex Analyzer; Radiometer Canada, Mississauga, ON, Canada) sampling perfusate from the arterial and portal venous inflows and IVC outflow every 2 hours, and as needed to maintain these parameters in physiologic range. Perfusate was also sampled from the IVC outflow, spun down to collect serum with an Eppendorf® 5180R centrifuge (1600 g for 15 min at 4ºC; Eppendorf North America, Inc., Farmington, MA, United States), and frozen at −80ºC for later measurement of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and total and conjugated bilirubin using an automated Beckman Coulter Unicel Dxc800 Syncron (Beckman Coulter Canada LP, Mississauga, ON, Canada) available at the clinical lab at the University of Alberta Hospital.
HISTOLOGY:
Incisional biopsies were taken from the right lateral lobe after 2, 6, and 12 hours of perfusion. A piece of tissue was fixed in 10% formalin, while the remainder was flash frozen in liquid nitrogen for tissue assays. Formalin-fixed tissue was then paraffin embedded, cut, and stained with hematoxylin and eosin (H&E). H&E slides were examined by a trained pathologist (AT) and scored based on the presence of necrosis, vacuolization, cholestasis, and congestion, as previously reported by Suzuki et al [17].
GLUTATHIONE & CYTOKINE ASSAYS:
Tissue glutathione was measured using a colorimetric assay kit (Invitrogen EIAGSHC, Fisher Scientific Co., Edmonton, AB, Canada), which was run according to the manufacturer’s instructions. Standards and samples were added in duplicate on a 96-well plate and, following completion of the reaction, absorbance at 405 nm was measured on a spectrophotometer (Multiskan SkyHigh; Fisher Scientific Co., Edmonton, AB, Canada). Levels of 11 different cytokines were measured in perfusate at 3 time points (0, 6, and 12 hours) using a porcine-specific multiplex assay (Discovery Assay® Array PD13) run by Eve Technologies Corporation based out of the University of Calgary.
STATISTICAL ANALYSIS:
Data are reported as medians (interquartile range). Normality was tested using the Shapiro-Wilk test and histogram distribution. Non-normal data were transformed using a process previously described [18]. The t test was used for comparisons between 2 groups. Ordinary one-way ANOVA tests were used for multiple group comparison, with Fisher’s least significant difference procedure applied for pairwise comparison in post hoc analysis. A P value of 0.05 was taken as significant. SPSS version 26 software (IBM Canada; Markham, ON, Canada) was used for statistical analysis.
Results
CSA TREATMENT HAD NO EFFECT ON LIVER PERFUSION PARAMETERS OR BILE PRODUCTION:
Perfusion parameters, including flow rate, pressure, and resistance are measured as a routine part of organ monitoring. Perfusion parameters were largely similar between groups (Figure 3). Peak HA flow was lower in the higher-ischemia group treated with CsA compared to the lower-ischemia control (330.3 mL/min [327.0–363.9] vs 433.2 mL/min [425.1–446.9]; P=0.016). However, minimum HA resistance was not different between groups (P=0.681). In contrast, peak PV flow was not different between groups (P=0.709), while minimum PV resistance was significantly higher in the higher-ischemia group treated with CsA compared to the lower-ischemia control (1.0 mmHg/min/mL [0.4–1.2] vs 0.3 mmHg/min/mL [0.1–0.4]; P=0.009). Median cumulative bile production was higher for livers in the lower-ischemia group (12.5mL [2.5–17.0] vs 2.5mL [2.0–18.0]), but this did not achieve statistical significance (P=0.277; Figure 4). No significant difference in bile production was seen between CsA-treated livers and controls at either degree of ischemia (P=0.987, 0.465 comparing control and treated livers in the lower and higher-ischemia levels, respectively).
PEAK LDH WAS DECREASED IN LIVERS TREATED WITH CSA AFTER 45 MIN OF WARM ISCHEMIA AND 2 H OF COLD ISCHEMIA:
Perfusate ALT, AST, and LDH were measured as markers of cellular damage. Peak LDH was significantly lower in the lower-ischemia treatment group (4220U/L [3515–5810]) compared to the other groups (11 305 U/L [10 100–11 674], 7939 U/L [6692–8760], 7992 U/L [7692–10 971]; P=0.023, 0.035, 0.019 for the lower-ischemia control and the higher-ischemia control and treated, respectively). However, peak values of perfusate ALT (which is more specific to the liver) for the lower-ischemia group treated with CsA (302U/L [191–314]) were no different from those of the other groups (334 U/L [254–334], 306 U/L [295–335], 313U/L [217–331]; P=0.519, 0.584, 0.747 for the lower-ischemia control and the higher-ischemia control and treated, respectively; Figure 5). Peak values of perfusate AST were similarly no different in the lower-ischemia treatment group (8265 U/L [4176–8660], 11305 U/L [10 100–11674], 7939 U/L [6692–8760], 7992 U/L [7692–10 971]; P=0.120, 0.382, 0.357 for the lower-ischemia control and the higher-ischemia control and treated, respectively).
END PH WAS DECREASED IN LIVERS WITH LESS ISCHEMIC INJURY, BUT WAS NOT AFFECTED BY CSA TREATMENT:
The liver plays a vital role in maintaining serum pH and regulating glycolysis, including metabolism of its main by-product, lactate [19]. As such, acidosis and rising perfusate lactate are indicative of poor liver function. We found pH at the end of perfusion was significantly decreased in the higher-ischemia groups compared to the lower-ischemia groups (7.346 [7.324–7.361] vs 7.407 [7.384–7.461]; P=0.001), despite having no difference in the amount of THAM required to maintain pH (42 mL [35–48] vs 45mL [33–50] for the lower- and higher-ischemia groups, respectively; P=0.605; Figure 6). However, there was no difference in end pH between the control and treated groups at either level of ischemia (P=0.752, 0.517 for the lower- and higher-ischemia groups, respectively). Perfusate lactate levels did not differ significantly over the course of the perfusion, nor were peak lactate levels different between groups (2.8 mmol/L [2.7–4.2], 3.3 mmol/L [2.1–5.1], 3.5 mmol/L [2.7–9.8], 4.1 mmol/L [2.8–6.8] for control and treated livers in the lower-ischemia group and control and treated livers in the higher-ischemia group, respectively (Figure 7).
:
Oxygen consumption is indicative of the metabolic activity of an organ. We found that peak oxygen consumption was also higher for livers in the lower-ischemia groups compared to those in the higher-ischemia groups (Figure 8). Peak oxygen consumption was 0.011 mL/min/g tissue (0.011–0.012) for the control and 0.014 mL/min/g tissue (0.009–0.016) for the treated groups at the higher-ischemia level compared to 0.017 mL/min/g tissue (0.015–0.018) for the control (P=0.029, 0.063 respectively) and 0.020 mL/min/g tissue (0.016–0.021) for the treated (P=0.007, 0.016 respectively) groups at the lower ischemia level. There was no difference, however, between control and CsA-treated groups with the same degree of ischemia (P=0.417, 0.689 for the lower and higher levels of ischemia, respectively).
HISTOLOGICAL INJURY SCORE WAS DECREASED AT THE END OF PERFUSION FOR LIVERS WITH LESS ISCHEMIC INJURY:
Total histological injury score was significantly lower for the lower-ischemia groups after 12 hours of perfusion compared to the higher-ischemia control (P=0.011, 0.027 for the lower injury control and treated groups, respectively). Histological sub-scores of sinusoidal congestion and necrosis were no different between groups at any of the time points. However, the vacuolization sub-score was significantly lower at 6 and 12 hours in the lower-ischemia groups compared to the higher-ischemia groups (P=0.019, <0.001 for 6 and 12 hours, respectively). Despite this, no difference in histological injury score was seen between control and CsA-treated groups at any of the time points for either degree of ischemia (Figure 9).
IL-10 WAS INCREASED IN THE PERFUSATE OF LIVERS TREATED WITH CSA AFTER 45 MIN OF WARM ISCHEMIA AND 2 H OF COLD ISCHEMIA:
Peak perfusate concentrations of the anti-inflammatory cytokine IL-10 were significantly increased in the lower-ischemia CsA-treated group (1608.92 pg/mL [1228.61–2497.26]) compared to the lower-ischemia control group and both of the higher-ischemia groups (451.61 pg/mL [386.26–548.88], 419.01 pg/mL [364.35–484.14], 391.72 pg/mL [270.34–527.34]; P=0.043, 0.042, 0.018 for lower-ischemia control and higher-ischemia control and treated groups, respectively). No difference was seen between groups in the peak concentrations of other inflammatory cytokines measured in the perfusate (Figure 10). Tissue glutathione, measured as a marker of oxidative stress, showed no difference between groups at any of the time points measured (Figure 11).
Discussion
In the present study, we tested the effect of CsA on hepatic IRI in a novel porcine DCD model using ex vivo perfusion. We found a minimal effect of CsA on reducing hepatic IRI at 2 different severities of ischemic injury. End pH and peak oxygen consumption were higher in the lower-ischemia livers, and the histological score at 12 hours was lower in the lower-ischemia livers compared to the higher-ischemia control group. However, these parameters were no different between control and treated livers within the same injury level. Only the peak LDH was lower in the CsA-treated group with lower ischemia, while the anti-inflammatory cytokine IL-10 was higher compared to the other groups.
CsA has shown promise as an agent to mitigate IRI in multiple preclinical studies, particularly in relation to cardiac ischemia [20]. Several preclinical studies of warm hepatic IRI have shown that pre-treatment with CsA can decrease serum transaminases several hours after reperfusion [8,9]. Indeed, in our own previous studies (currently under review elsewhere), we found that mice treated with doses of CsA greater than 10 mg/kg had significant reduction in serum ALT, as well as decreased histological evidence of injury after 6 hours of reperfusion. We additionally found a similar effect with a unique CsA analog called NIM-811, which lacks immunosuppressive capacity, suggesting that this protective mechanism is unrelated to its immunosuppressive effect. We found marked reduction in apoptosis in the mouse studies, and suppression of the cytokine IL1-beta. Studies of the effect of CsA on warm hepatic IRI in porcine models have shown improved survival with pre-administration [10]. Less is known about the effect of CsA on liver IRI in the setting of combined warm and cold ischemia, as occurs in liver transplantation. The few rat liver transplantation studies that exist suggest that CsA may improve survival if given to the recipient prior to transplant following up to 12 hours of SCS, but may be less effective if given to the donor only [11,12]. Donor pre-treatment is currently the subject of a clinical trial in France and potentially in Canada in human organ donors (ClinicalTrials.gov NCT02907554).
There are several potential reasons why CsA was not effective at reducing IRI in our model. The dose of 20 mg/kg was chosen based on a review of literature, including both hepatic and non-hepatic models, as well as our own previous positive experience in a murine model of hepatic injury [20]. The most common dose administered was 10 mg/kg. A higher dose was chosen for the present study, as we observed that studies including porcine and/or female animals tended to reported weaker effects [20]. Indeed, the serum level of CsA at the time of hepatectomy was above the limit of detection at our clinical lab (5000 ng/mL) for all animals treated with CsA, so it is unlikely that dose was the issue. It may be that CsA is less effective in pigs compared to rats or less effective in female animals. Pigs were used in our model owing to their physiologic similarity to humans and, for reasons of size and husbandry, female pigs are more easily obtained in our setting (male animals are typically neutered by this age and size to limit their aggressiveness) [21].
It is possible that the duration of ischemia in our model was too long and excessively severe and that CsA would be effective with shorter ischemic times (either warm or cold ischemia). We chose warm ischemic times of 45 and 60 minutes to reflect the upper limits of what would be tolerated clinically (also considering that these are young, healthy animals). Knaak et al published a similar DCD model employing 45 minutes of warm ischemia and 4 hours of SCS [22]. Our use of a whole-blood-base perfusate in a normothermic setting (37ºC), rather than diluted filtered red blood cells at subnormothermic temperatures (33ºC) may explain why we observed a more severe injury with similar ischemic times. In addition, CsA may be more effective in reducing injury from warm ischemia, rather than cold ischemia (or both, as in our model). A study by Tarrab et al using a model of rat liver transplantation found that CsA given to the recipient was not protective against IRI following prolonged SCS (24 hours) [12]. This would certainly limit the utility of CsA in the setting of organ transplantation, given the obligatory period of cold ischemia.
The end points for our study were chosen to reflect well-understood clinical parameters of liver injury, as our intention was to evaluate whether CsA would be a suitable clinical intervention to mitigate IRI in the context of liver transplantation. The specific mechanism by which CsA mitigates IRI has been shown in several other studies and was not the aim of this specific study, although in our murine studies we found marked suppression of apoptotic injury. Given that we did not find an effect by any clinically meaningful parameters, we opted not to pursue further investigations.
An additional major limitation to our study is that the liver was not transplanted into a live animal for reperfusion. Reperfusion in the ex vivo setting allows for a potentially longer reperfusion phase (particularly in an injury model) and is less labor-intensive (as transplantation of a large animal requires invasive monitoring and may not be recoverable). However, ex vivo reperfusion lacks the interaction with other organ systems that can also play a role in IRI [23,24]. We fully acknowledge that with only 5 pigs per group (split over 4 groups) predictive interpretation is clearly limited, and a larger study might have found a more subtle but relevant protective benefit.
Conclusions
Overall, our study found only a minimal benefit of pre-treatment with CsA to prevent IRI in a large-animal DCD model. Despite some studies showing CsA to be protective in warm hepatic IRI, we did not find it to be beneficial in preventing injury following combined warm and cold ischemia. However, our model is novel in its use of whole-blood-based ex vivo perfusion to induce IRI in an isolated liver and may serve as a basis for testing other compounds that may be beneficial in preventing IRI in the setting of liver transplantation.
Figures
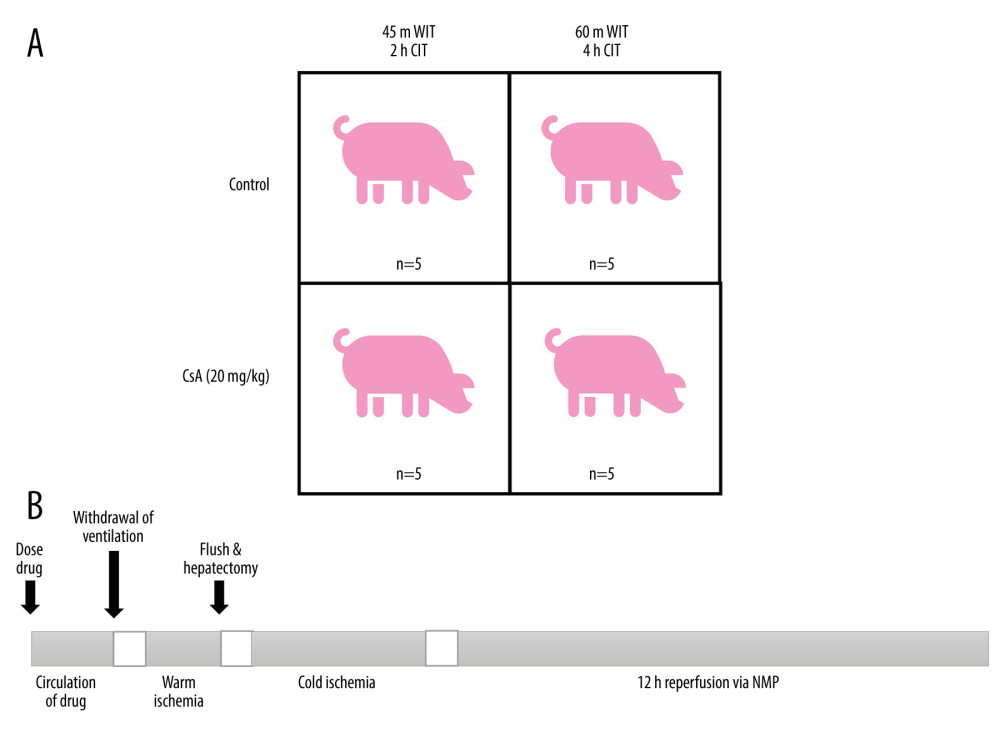 Figure 1. Study groups treated with normal saline (Ctrl) or cyclosporine A (CsA) and subject to either 45 minutes of warm ischemia (WIT), followed by 2 hours of cold ischemia (CIT) or 60 minutes of WIT followed by 4 hours of CIT (A). Outline of experimental procedure (B). Created in PowerPoint (v17.0, Microsoft Corp.).
Figure 1. Study groups treated with normal saline (Ctrl) or cyclosporine A (CsA) and subject to either 45 minutes of warm ischemia (WIT), followed by 2 hours of cold ischemia (CIT) or 60 minutes of WIT followed by 4 hours of CIT (A). Outline of experimental procedure (B). Created in PowerPoint (v17.0, Microsoft Corp.). 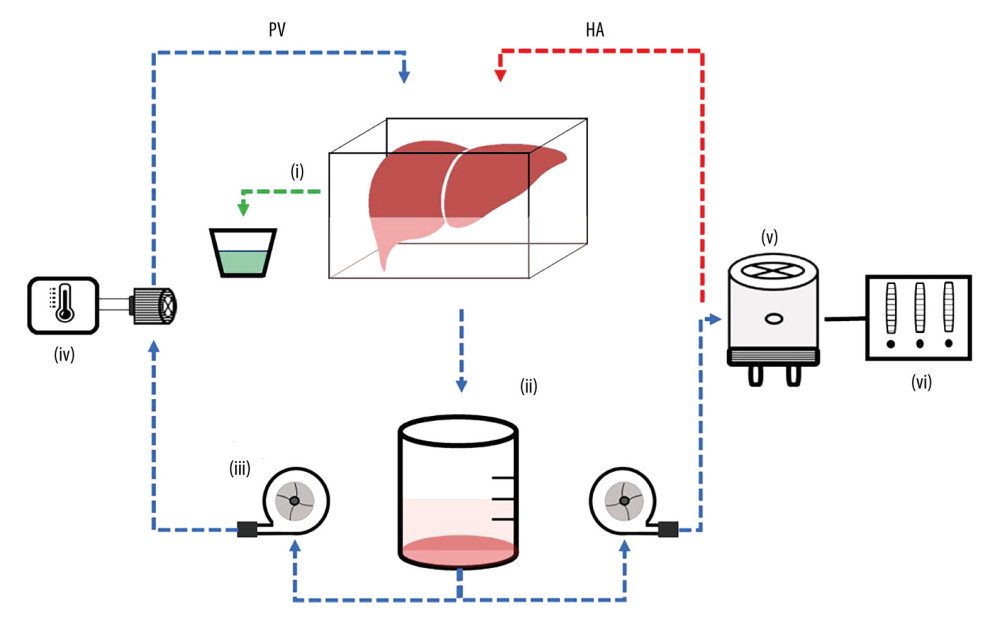 Figure 2. Liver ex vivo normothermic machine perfusion schematic. Reprinted with permission from Hefler et al. Biomedicines (2022) Oct 6;10(10): 2496. Created in PowerPoint (v19.0, Microsoft Corp.).
Figure 2. Liver ex vivo normothermic machine perfusion schematic. Reprinted with permission from Hefler et al. Biomedicines (2022) Oct 6;10(10): 2496. Created in PowerPoint (v19.0, Microsoft Corp.). 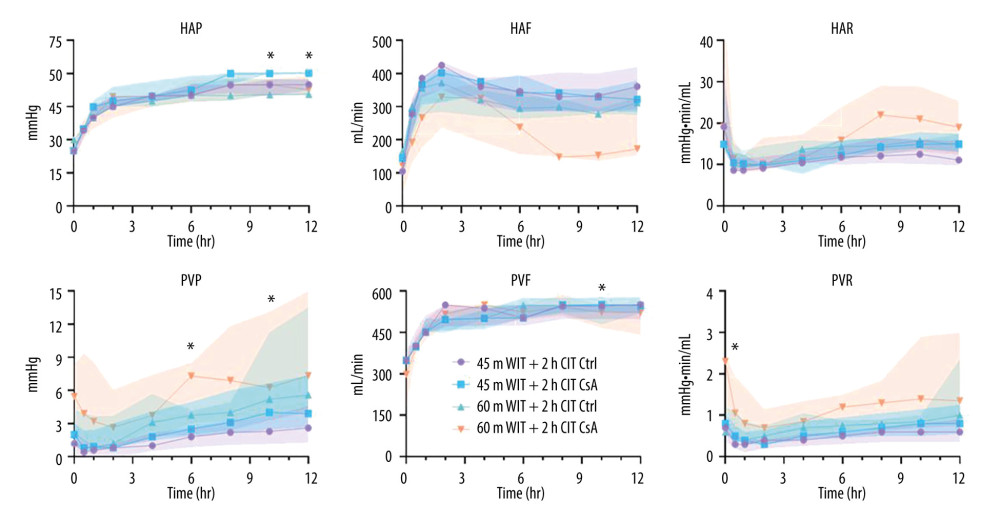 Figure 3. Perfusion parameters for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v17.0, GraphPad Software Inc.). HAP – hepatic artery pressure; HAF – hepatic artery flow; HAR – hepatic artery resistance; PVP – portal venous pressure; PVF – portal venous flow; PVR – portal venous resistance.
Figure 3. Perfusion parameters for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v17.0, GraphPad Software Inc.). HAP – hepatic artery pressure; HAF – hepatic artery flow; HAR – hepatic artery resistance; PVP – portal venous pressure; PVF – portal venous flow; PVR – portal venous resistance. 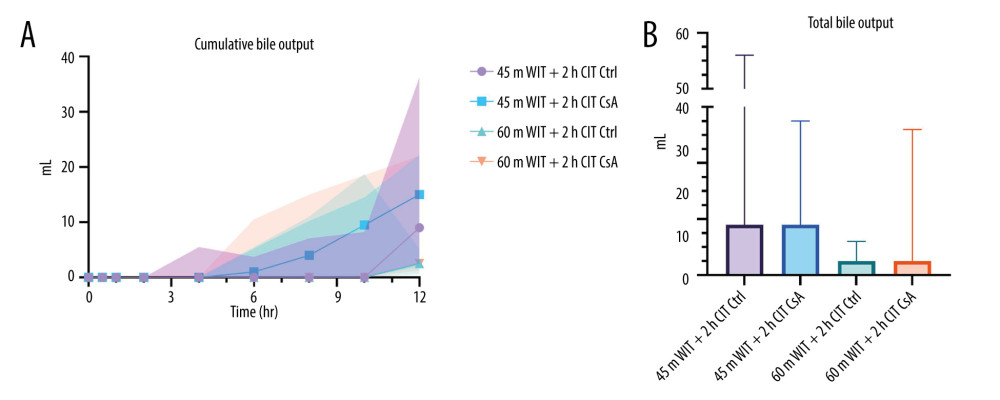 Figure 4. Cumulative (A) and total (B) bile output over 12 hours reperfusion for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 4. Cumulative (A) and total (B) bile output over 12 hours reperfusion for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). 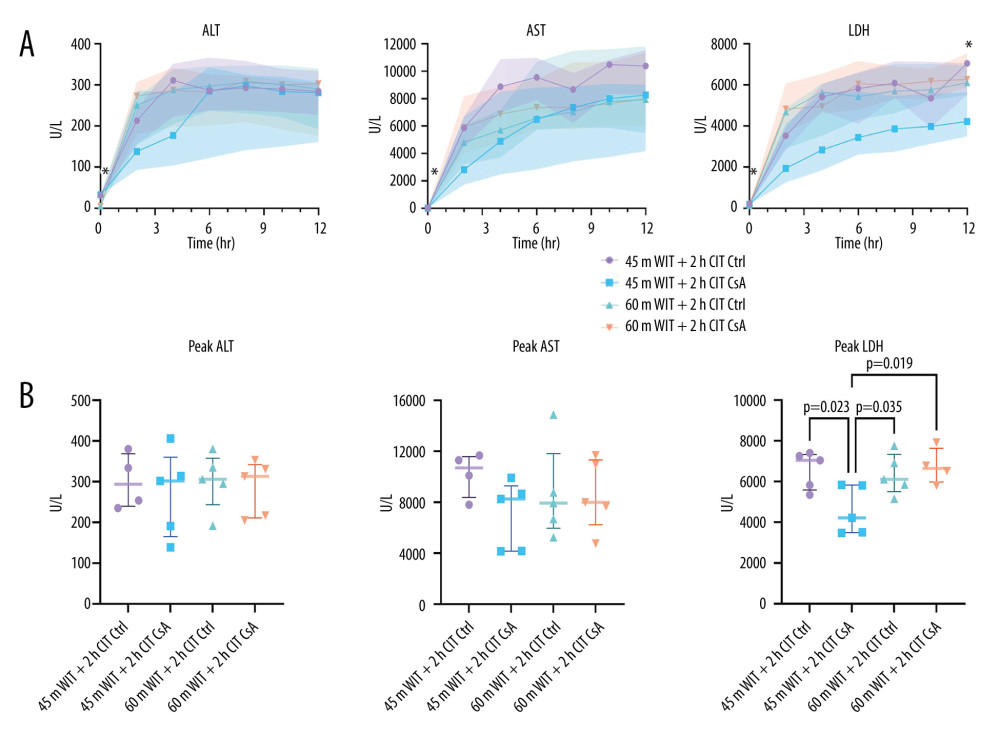 Figure 5. Perfusate levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) over the course of reperfusion (A) and at their peak (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 5. Perfusate levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) over the course of reperfusion (A) and at their peak (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). 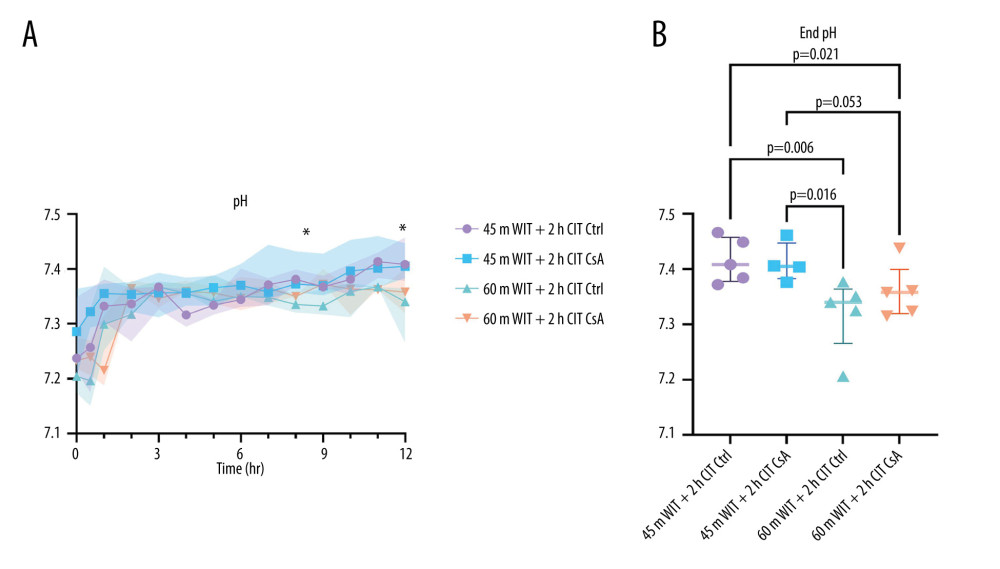 Figure 6. Perfusate pH over the course of reperfusion (A) and at the end (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 6. Perfusate pH over the course of reperfusion (A) and at the end (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). 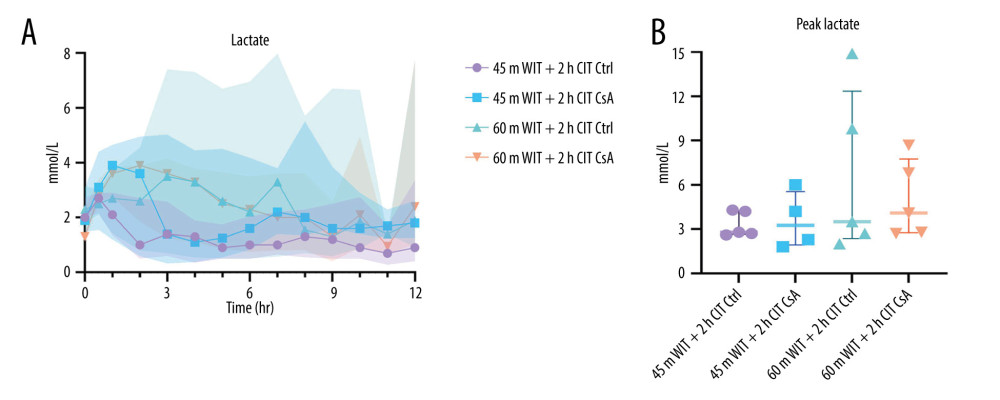 Figure 7. Perfusate lactate over the course of reperfusion (A) and at peak levels (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 7. Perfusate lactate over the course of reperfusion (A) and at peak levels (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). 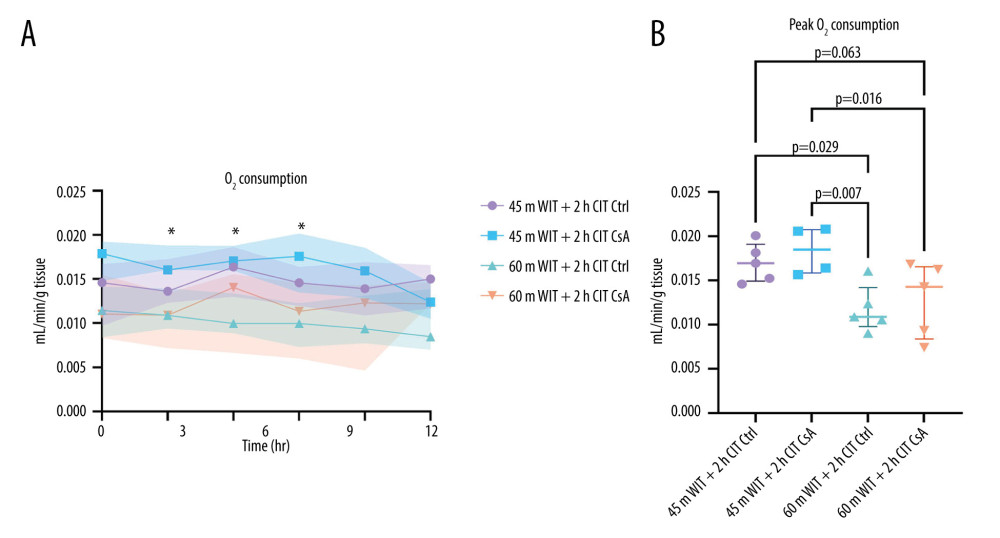 Figure 8. Oxygen (O2) consumption over the over the course of reperfusion (A) and at peak levels (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 Hh CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 8. Oxygen (O2) consumption over the over the course of reperfusion (A) and at peak levels (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 Hh CIT). Created in Prism (v9.0, GraphPad Software, Inc.). 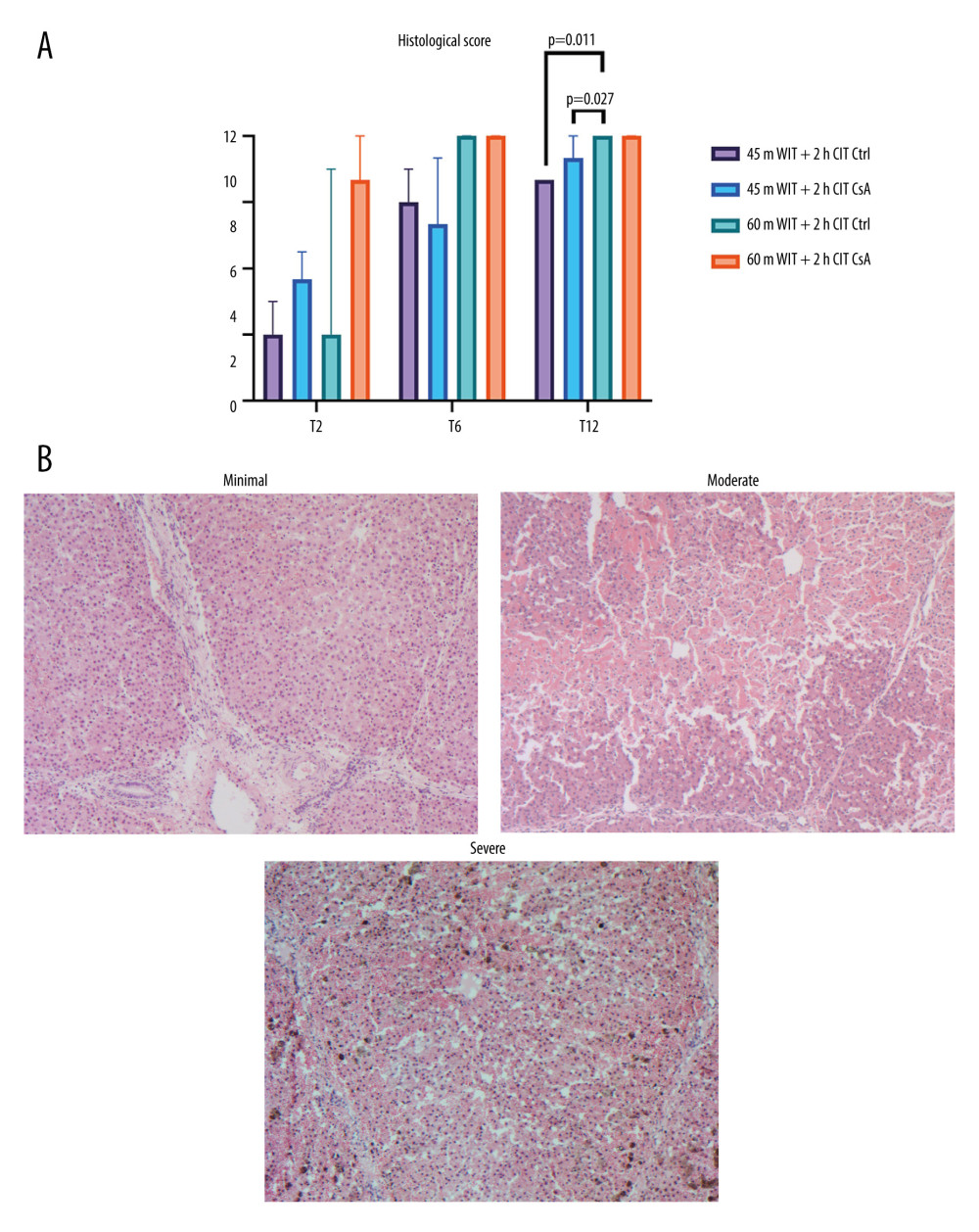 Figure 9. Histological injury scores for biopsies taken at 2 (T2), 6 (T6), and 12 (T12) hours (A) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Representative images of minimal, moderate, and severe injury taken at 50× magnification (B). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 9. Histological injury scores for biopsies taken at 2 (T2), 6 (T6), and 12 (T12) hours (A) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Representative images of minimal, moderate, and severe injury taken at 50× magnification (B). Created in Prism (v9.0, GraphPad Software, Inc.). 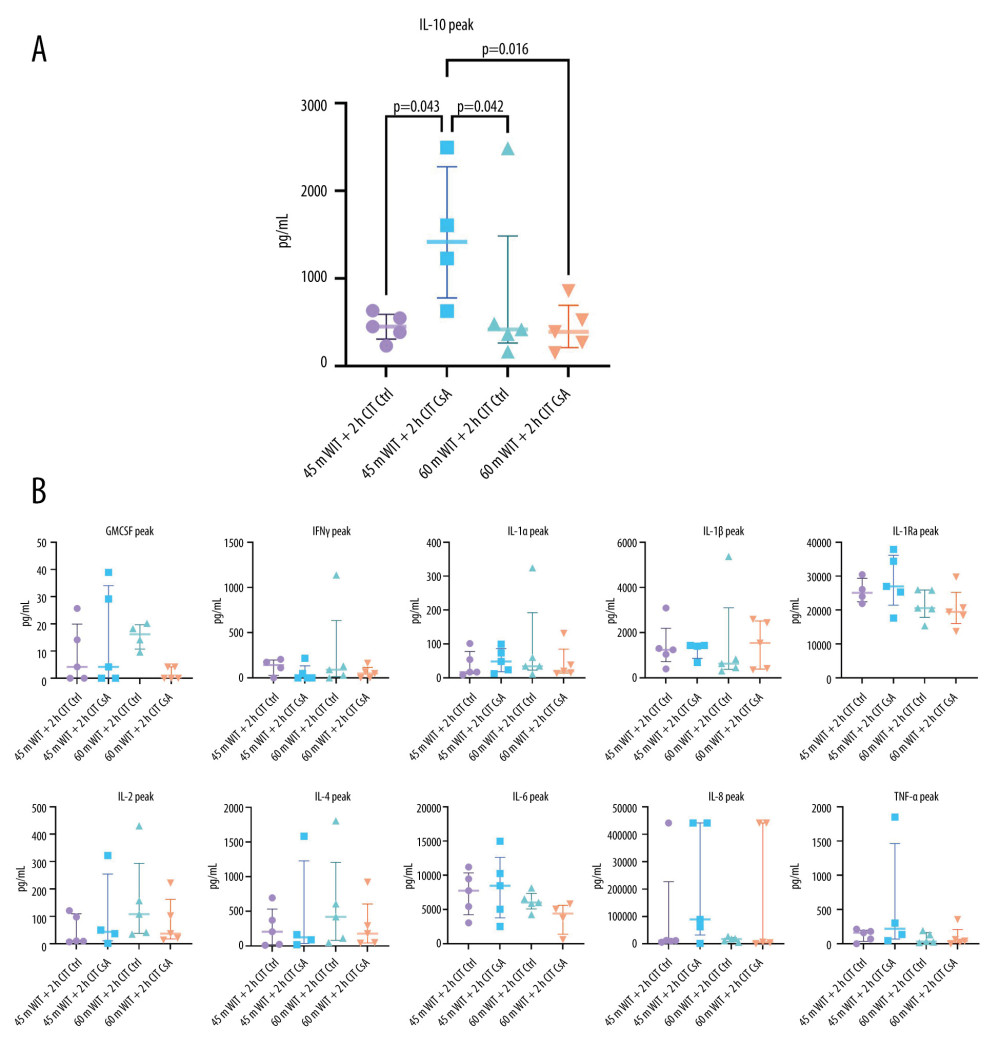 Figure 10. (A, B) Peak levels of perfusate cytokines for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). GMCSF – granulocyte macrophage colony stimulating factor; IFNγ – interferon gamma; IL – interleukin; TNFα – tissue necrosis factor alpha.
Figure 10. (A, B) Peak levels of perfusate cytokines for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). GMCSF – granulocyte macrophage colony stimulating factor; IFNγ – interferon gamma; IL – interleukin; TNFα – tissue necrosis factor alpha. 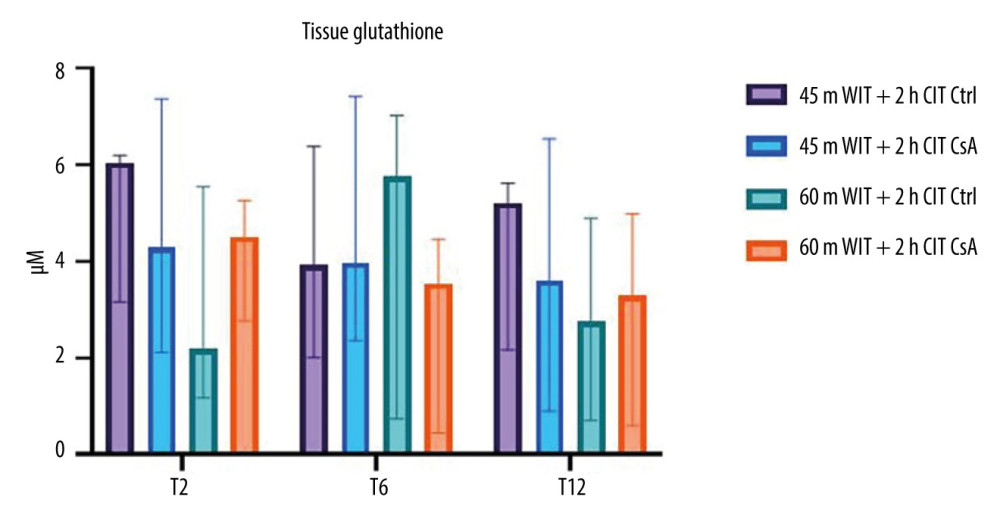 Figure 11. Tissue glutathione concentrations for liver biopsies taken at 2 (T2), 6 (T6), and 12 (T12) hours after reperfusion for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.
Figure 11. Tissue glutathione concentrations for liver biopsies taken at 2 (T2), 6 (T6), and 12 (T12) hours after reperfusion for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc. References
1. Cowled P, Fitridge R, Pathophysiology of reperfusion injury: Mechanisms of vascular disease: A reference book for cascular specialists [Internet], 2011, Adelaide (AU), University of Adelaide Press [cited 2023 Mar 29]. Available from:http://www.ncbi.nlm.nih.gov/books/NBK534267/
2. Wu MY, Yiang GT, Liao WT, Current mechanistic concepts in ischemia and reperfusion injury: Cell Physiol Biochem, 2018; 46(4); 1650-67
3. Yuan X, Theruvath AJ, Ge X, Machine perfusion or cold storage in organ transplantation: Indication, mechanisms, and future perspectives: Transpl Int, 2010; 23(6); 561-70
4. Thuong M, Ruiz A, Evrard P, New classification of donation after circulatory death donors definitions and terminology: Transpl Int, 2016; 29(7); 749-59
5. Kubal C, Roll GR, Ekser B, Muiesan P, Donation after circulatory death liver transplantation: What are the limits for an acceptable DCD graft?: Int J Surg, 2020; 82S; 36-43
6. Patocka J, Nepovimova E, Kuca K, Wu W, Cyclosporine A: Chemistry and toxicity – A review: Curr Med Chem, 2021; 28(20); 3925-34
7. Giorgio V, Soriano ME, Basso E, Cyclophilin D in mitochondrial pathophysiology: Biochim Biophys Acta, 2010; 1797(6–7); 1113-18
8. Eum HA, Cha YN, Lee SM, Necrosis and apoptosis: Sequence of liver damage following reperfusion after 60 min ischemia in rats: Biochem Biophys Res Commun, 2007; 358(2); 500-5
9. Hirakawa A, Takeyama N, Nakatani T, Tanaka T, Mitochondrial permeability transition and cytochrome c release in ischemia-reperfusion injury of the rat liver: J Surg Res, 2003; 111(2); 240-47
10. Kim YI, Akizuki S, Kawano K, Goto S, Intrahepatic neutrophil accumulation in ischemia/reperfusion injury of pig liver: Transplant Proc, 1994; 26(4); 2392-94
11. Goto S, Kim YI, Shimada T, Kawano K, Kobayashi M, The effects of pretransplant cyclosporine therapy on rats grafted with twelve-hour cold-stored livers – with special reference to reperfusion injury: Transplantation, 1991; 52(4); 615-21
12. Tarrab E, Huet PM, Brault A, Cyclosporin-A does not prevent cold ischemia/reperfusion injury of rat livers: J Surg Res, 2012; 175(2); 333-42
13. Charan J, Kantharia ND, How to calculate sample size in animal studies?: J Pharmacol Pharmacother, 2013; 4(4); 303-6
14. Bral M, Gala-Lopez B, Thiesen A, Determination of minimal hemoglobin level necessary for normothermic porcine ex situ liver perfusion: Transplantation, 2018; 102(8); 1284-92
15. Nostedt JJ, Churchill T, Ghosh S, Avoiding initial hypothermia does not improve liver graft quality in a porcine donation after circulatory death (DCD) model of normothermic perfusion: PLoS One, 2019; 14(8); e0220786
16. Swindle MM, Liver and biliary system: Swine in the Laboratory, 2016; 135-54, CRC Press
17. Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D, Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine: Transplantation, 1993; 55(6); 1265-72
18. Templeton G, A two-step approach for transforming continuous variables to normal: Implications and recommendations for IS research: Communications of the Association for Information Systems, 2011; 28(1); 41-58
19. Kamel KS, Oh MS, Halperin ML, L-lactic acidosis: pathophysiology, classification, and causes; emphasis on biochemical and metabolic basis: Kidney Int, 2020; 97(1); 75-88
20. Hefler J, Marfil-Garza BA, Campbell S, Preclinical systematic review & meta-analysis of cyclosporine for the treatment of myocardial ischemia-reperfusion injury: Ann Transl Med, 2022; 10(18); 954
21. Lunney JK, Van Goor A, Walker KE, Importance of the pig as a human biomedical model: Sci Transl Med, 2021; 13(621); eabd5758
22. Knaak JM, Spetzler VN, Goldaracena N, Technique of subnormothermic ex vivo liver perfusion for the storage, assessment, and repair of marginal liver grafts: J Vis Exp, 2014(90); e51419
23. Ali M, Pham A, Wang X, Extracellular vesicles for treatment of solid organ ischemia-reperfusion injury: Am J Transplant, 2020; 20(12); 3294-307
24. Shang Y, Madduma Hewage S, Wijerathne CUB, Kidney Ischemia-reperfusion elicits acute liver injury and inflammatory response: Front Med, 2020; 7; 201
Figures
 Figure 1. Study groups treated with normal saline (Ctrl) or cyclosporine A (CsA) and subject to either 45 minutes of warm ischemia (WIT), followed by 2 hours of cold ischemia (CIT) or 60 minutes of WIT followed by 4 hours of CIT (A). Outline of experimental procedure (B). Created in PowerPoint (v17.0, Microsoft Corp.).
Figure 1. Study groups treated with normal saline (Ctrl) or cyclosporine A (CsA) and subject to either 45 minutes of warm ischemia (WIT), followed by 2 hours of cold ischemia (CIT) or 60 minutes of WIT followed by 4 hours of CIT (A). Outline of experimental procedure (B). Created in PowerPoint (v17.0, Microsoft Corp.). Figure 2. Liver ex vivo normothermic machine perfusion schematic. Reprinted with permission from Hefler et al. Biomedicines (2022) Oct 6;10(10): 2496. Created in PowerPoint (v19.0, Microsoft Corp.).
Figure 2. Liver ex vivo normothermic machine perfusion schematic. Reprinted with permission from Hefler et al. Biomedicines (2022) Oct 6;10(10): 2496. Created in PowerPoint (v19.0, Microsoft Corp.). Figure 3. Perfusion parameters for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v17.0, GraphPad Software Inc.). HAP – hepatic artery pressure; HAF – hepatic artery flow; HAR – hepatic artery resistance; PVP – portal venous pressure; PVF – portal venous flow; PVR – portal venous resistance.
Figure 3. Perfusion parameters for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v17.0, GraphPad Software Inc.). HAP – hepatic artery pressure; HAF – hepatic artery flow; HAR – hepatic artery resistance; PVP – portal venous pressure; PVF – portal venous flow; PVR – portal venous resistance. Figure 4. Cumulative (A) and total (B) bile output over 12 hours reperfusion for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 4. Cumulative (A) and total (B) bile output over 12 hours reperfusion for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). Figure 5. Perfusate levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) over the course of reperfusion (A) and at their peak (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 5. Perfusate levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) over the course of reperfusion (A) and at their peak (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). Figure 6. Perfusate pH over the course of reperfusion (A) and at the end (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 6. Perfusate pH over the course of reperfusion (A) and at the end (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). Figure 7. Perfusate lactate over the course of reperfusion (A) and at peak levels (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 7. Perfusate lactate over the course of reperfusion (A) and at peak levels (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). Figure 8. Oxygen (O2) consumption over the over the course of reperfusion (A) and at peak levels (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 Hh CIT). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 8. Oxygen (O2) consumption over the over the course of reperfusion (A) and at peak levels (B) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 Hh CIT). Created in Prism (v9.0, GraphPad Software, Inc.). Figure 9. Histological injury scores for biopsies taken at 2 (T2), 6 (T6), and 12 (T12) hours (A) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Representative images of minimal, moderate, and severe injury taken at 50× magnification (B). Created in Prism (v9.0, GraphPad Software, Inc.).
Figure 9. Histological injury scores for biopsies taken at 2 (T2), 6 (T6), and 12 (T12) hours (A) for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Representative images of minimal, moderate, and severe injury taken at 50× magnification (B). Created in Prism (v9.0, GraphPad Software, Inc.). Figure 10. (A, B) Peak levels of perfusate cytokines for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). GMCSF – granulocyte macrophage colony stimulating factor; IFNγ – interferon gamma; IL – interleukin; TNFα – tissue necrosis factor alpha.
Figure 10. (A, B) Peak levels of perfusate cytokines for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.). GMCSF – granulocyte macrophage colony stimulating factor; IFNγ – interferon gamma; IL – interleukin; TNFα – tissue necrosis factor alpha. Figure 11. Tissue glutathione concentrations for liver biopsies taken at 2 (T2), 6 (T6), and 12 (T12) hours after reperfusion for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc.
Figure 11. Tissue glutathione concentrations for liver biopsies taken at 2 (T2), 6 (T6), and 12 (T12) hours after reperfusion for control (Ctrl) and treated (CsA) livers subject to 45 or 60 minutes of warm ischemia (45 m WIT, 60 m WIT) and 2 or 4 hours of cold ischemia (2 h CIT, 4 h CIT). Created in Prism (v9.0, GraphPad Software, Inc. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








