28 November 2023: Original Paper
Toll-Like Receptor 3 mRNA Expression of Peripheral Blood Mononuclear Cells Identifies Kidney Recipients with Potential for Improved Graft Performance
Sławomir C. ZmonarskiDOI: 10.12659/AOT.941266
Ann Transplant 2023; 28:e941266
Abstract
BACKGROUND: Toll-like receptor 3 expression is detected both on the cell membrane and in endosomes of peripheral blood mononuclear cells (PBMC). Our goal in this study was to determine to what extent a single, baseline measurement of non-stimulated PBMC TLR3-mRNA can be related to baseline GFR (b-GFR) and post-follow-up-GFR (F-up-GFR) of a kidney transplant (KT) and baseline immunosuppression.
MATERIAL AND METHODS: In non-stimulated PBMC we investigated averaged mRNA expression of Toll-like receptor 3. A total of 133 patients were enrolled; the median of months after KT surgery was 11.4, with median F-up at 21.3 months. A favorable course (FCF) was determined if F-up-eGFR improved. An unfavorable course (UCF) was determined if F-up-eGFR was lower at the end of the observation.
RESULTS: The highest TLR3-mRNA expression was at b-GFR grade 3b; it was moderately higher at b-GFR grade 3a, and marginally higher at b-GFR grades 1+2. Most of the FCF group had b-GFR grade 3b, less frequent obesity, more effective immunosuppression, and much higher TLR3-mRNA (59% of cases were in the high-TLR3 area). Both delayed graft function (DGF) and TLR3-mRNA range below the median for the entire KT cohort (low-TLR3 area) had a negative association with b-GFR. The UCF group had more frequent DGFs and obesity, less effective immunosuppression, and lower TLR3-mRNA.
CONCLUSIONS: In patients with GFR grade 3, high levels of TLR3-mRNA are associated with improved graft efficacy. In patients with impaired graft function, low TLR3- mRNA expression reduces the likelihood of improved renal graft function.
Keywords: RNA, Messenger, TLR3 Protein, Human, renal insufficiency, Kidney Transplantation, Leukocytes, Mononuclear
Background
Toll-like receptor (TLR) 3 is a type I transmembrane protein that can recognize double-stranded RNA (ds-RNA) or synthetic ds-RNA analogs [1–4]. The source of ds-RNA can be damaged cells, which are primarily destroyed through necroptosis [1,4–6]. Necroptosis can occur in ischemia-reperfusion injury or in solid-organ transplant rejection [1,2,5]. Toll-like receptor 3 plays an important role in the recognition of conserved molecules that are part of the pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) [7]. Tissue regeneration and wound healing both are critically dependent on TLR-mediated cellular activity, but Toll-like receptor 3 also plays a key role in tissue damage [8,9]. The receptor can be found in various somatic normal and neoplastic cells [1,2,8–10] and in several subsets of immune cells [1,2,4,11–13]. At rest, Toll-like receptor 3 expression may be very low. T-receptor stimulation markedly increases Toll-like receptor 3 expression [8].
It has been traditionally assumed that intracellular organelles are the dominant localization sites for Toll-like receptor 3. Nonetheless, an increasing number of studies prove the presence of Toll-like receptor 3 on the surface of the cell membrane [1,2,13,14]. It is likely that Toll-like receptor 3 is present as a functional receptor on the cell membrane more often than other endosomal TLRs [14]. Surface receptors are assigned an important role in detecting exogenous ds-RNA as well as initiating and controlling a response that increases endosomal Toll-like receptor 3 expression [4,14]. The effect of efficient stimulation of Toll-like receptor 3 is the synthesis of biochemical inflammatory mediators, including interferons (IFN) t. 1 and the expression of a number of enzymes, mainly caspases [1–3,15,16]. In some somatic cells [12] and in leukocytes and tissue macrophages [5,12,15] and dendritic cells, [11] strong stimulation of Toll-like receptor 3 can lead to apoptosis or necroptosis, which facilitates recruitment of inflammatory cells to the site of infection or cell damage [2,5,14]. Toll-like receptor 3 polymorphisms or polymorphisms of the post-receptor transmission pathway can affect receptor expression and reduce the risk of acute rejection [7].
Clinical evaluation of the prognosis in renal transplant recipients is usually based on long-term clinical observations, such as scheduled laboratory tests and physical examinations. Our previous publications on mRNA of Toll-like receptor 4 expression have shown that there is a non-linear association between expression of TLR4-mRNA and renal transplant performance, and that this relationship is influenced by previously administered therapeutic interventions and current immunosuppression [17,18]. We believe that there are similar associations with TLR3-mRNA of PBMC. Unlike dendritic and mast cells, peripheral blood mononuclear cells are readily available and their separation is technologically much simpler. Our main goal was to determine to what extent a single measurement of TLR3-mRNA expression is related to the current and predicted performance of a kidney transplant. The second goal was to assess the extent to which very basic clinical parameters connected with graft performance and current immunosuppression are related to TLR3-mRNA expression.
Material and Methods
MATERIAL:
The study population was recruited from patients who underwent kidney transplantation (KT) between the years 2012 and 2019 and were registered with a transplant outpatient clinic. The precondition for inclusion in the study was at least 30 days from the date of kidney transplantation. The criterion of delayed graft function (DGF) was the need for at least 1 hemodialysis session since the third day after KT. All patients were treated with oral prednisone. In all patients, cyclosporine A/tacrolimus-dependent immunosuppression remained stable at least 1 week before day 0 and during the observation period (follow-up) with fluctuations in drug levels not greater than ±10% from baseline [19,20]. A sex- and age-matched control group without any known systemic disorder was also recruited. Table 1 presents the basic data for the study groups.
The investigations were carried out following the rules of the Declaration of Helsinki of 1975 (
METHODS:
The start date of observation (study start) was called the “baseline” (median 11.15 months, 3.84–42.8 from transplant surgery). On the first (baseline) day of observation, a detailed medical history was taken and blood was collected for basic tests and mRNA expression analysis. In non-stimulated PBMC we investigated averaged mRNA expression of Toll-like receptors 3 using real-time polymerase chain reaction (RT-PCR). The method evaluated the entire TLR3-mRNA pool. PBMCs were separated from heparinized blood samples, mRNA was isolated with the RNeasy Mini Kit (QIAGEN), and reversely transcribed with a High-Capacity RNA to cDNA kit (Applied Biosystems). The cDNA amount for TLR genes and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was assessed using the TaqMan 9700HT real-time PCR system. All the procedures were performed according to the manufacturer’s protocols. The data of the control group (Contr., 46 volunteers) were used only for PCR data normalization. Expression data are presented as ΔΔCt=mean (ΔCtContr-ΔCtSample), where ΔCt=Ctgene-CtGAPDH. “Ct” is the cycle threshold value and defines the calculated number of cycles in which the fluorescence measured during the PCR reaction increases over the preset threshold value. The ΔΔCt value of a given patient shows the difference in the mean TLR3-mRNA expression of his PBMC population relative to the corresponding mean TLR3-mRNA expression in the control group. The ΔΔCt for the different groups shows the median of the set of individual ΔΔCts. In our opinion, the advantage of evaluating TLR3 mRNA expression is its relation to both endosomal and surface TLR3 receptors. For some analyses, the entire cohort of transplant recipients had to be divided into 2 groups: the one with a lower TLR3-mRNA expression range (below the median for the whole cohort, Low-TLR3-mRNA,) and the one with a higher TLR3-mRNA expression range (above the median for the entire cohort, High-TLR3-mRNA) [17,21] (Table 1). When we use the terms cyclosporin (CsA) and tacrolimus (Tac) concentration, this applies to a blood sample taken on initial the day just before the morning dose [17,21]. All renal biopsies were performed approximately 6 months (median) before inclusion in the study (historical biopsy) “on clinical indication” (Table 1).
STATISTICAL ANALYSIS:
Due to the predominant lack of normality of the data, results are expressed as the median and interquartile range (x, y to z), unless otherwise specified. Chi-square tests were applied to categorical variables. The comparison of quantitative variables between different study groups was performed using the Mann-Whitney test. Change in related baseline renal function parameters (estimated glomerular filtration rate – eGFR) was assessed using the signed rank and Wilcoxon tests. Regression analyses were performed using the quick regression method [22]. Qualitative predictors were verified with a post hoc analysis using Tukey’s honestly significant differences (HSD) test. P value <0.05 was considered statistically significant, with P<0.1 deemed a marginally significant difference. Statistical analyses were performed using the Statistica 13a PL software accessed using the Medical University’s academic license [17,21].
Results
GENERAL OVERVIEW OF THE ENTIRE PATIENT COHORT AT BASELINE (ALL PATIENTS):
The median expression of TLR3 mRNA for the entire study population (−0.51) was shifted downwards relative to the corresponding value for the control population. Except for 1 patient, all patients had at least mildly impaired baseline graft function. We found very low TLR3-mRNA expression in recipients with estimated glomerular filtration rate (eGFR) <30 ml/min (GFR grade 4 and 5). Among all recipients, the highest expression of TLR3-mRNA was noted in those with eGFR in the range of 30–45 ml/min (GFR grade 3b), moderately higher in those with eGFR at 45–60 ml/min (GFR grade 3a), and marginally higher in those with eGFR >60 ml/min (GFR grade 1+2) compared to recipients with GFR grade 4+5 (Table 2). Regression analysis showed that baseline eGFR was affected by historical Banff “t” values greater than zero and those TLR3-mRNA expression values that were over the median for the entire cohort (high TLR3-mRNA expression range, High-TLR3) (Table 3). Overall, we found only a weak (marginal) inverted relationship between the occurrence of abnormal values (normal vs abnormal) of some parameters of the semi-quantitative assessment of the historical kidney biopsy (Banff: “g”, “v”) and TLR3-mRNA expression. However, in the Low-TLR3 group, there was a clear inverse association between abnormal Banff “t” and “v” and TLR3-mRNA expression. We did not find any such relationship in the High-TLR3 group.
We found no relationship between cold ischemia time and TLR3-mRNA expression, but we noticed a negative association of delayed graft function duration with baseline TLR3-mRNA expression (Table 3). Strong correlations between kidney transplant surgery (KT) up to the day 0 – time factor and concentrations of both CsA (R=0.77, P<0.001) and Tac (R=0.67; P<0.001) were noticed. In this way, for our analyses, kidney transplant surgery up to the baseline - time factor has been considered as a dependent factor [17,21]. In the whole cohort, we found no clear association of tacrolimus or cyclosporin (in milligrams per kilogram body weight or blood concentration, respectively) with TLR3-mRNA expression. Regarding glucocorticoid treatment, only among tacrolimus-treated recipients was there a clear negative relationship between the cumulative dose of intravenous methylprednisolone and baseline TLR3-mRNA expression.
FOLLOW-UP: FAVORABLE COURSE OF OBSERVATION (FCF) VS UNFAVORABLE COURSE OF OBSERVATION (UCF):
The course of follow-up was considered favorable (FCF) if the eGFR values (follow-up eGFR) improved compared to the baseline after its completion. It was assumed that the function of the transplanted kidney worsened (UCF) when follow-up eGFR fell at the end of the observation period.
At the beginning of the follow-up period, the baseline expression of TLR3-mRNA in the FCF group was higher than in the UCF (Table 2). In the FCF group, 59.3% of the graft recipients had baseline TLR3-mRNA expression above the median for the overall patient cohort (high TLR3-mRNA) – chi-square=5.38; P=0.024. In the general cohort, we found a mild association of the baseline high TLR3-mRNA expression with eGFR after the follow-up period (Table 4). No differences in baseline eGFR values were found between the UCF group and the FCF group (Table 2). In FCF, we found both positive associations between baseline eGFR and TLR3-mRNA expression and between the baseline TLR3-mRNA expression and follow-up eGFR (Table 4). Tests for related observations showed that improvements in graft function can only be expected in patients expressing TLR3-mRNA above the median for the whole cohort (High-TLR, a qualitative parameter). A more detailed analysis showed that in the High-TLR3 group, the improvement in graft function was found almost exclusively in patients with a baseline eGFR in the range of 30–45 ml/min (grade 3b) (Table 5). There was no significant difference between the FCF and UCF groups in the length of prospective follow-up of patients. Moreover, in kidney recipients with a baseline GFR >60 ml/min (grade 1 or 2 GFR), TLR3 mRNA expression at baseline was higher in the group that had improved graft function during follow-up (FCF60+) than in the control population and much higher than in the group in which graft function deteriorated (UCF60+) (Table 2).
There were no differences in the distribution of baseline tacrolimus blood concentrations (P=0.27) between UCF and FCF. The FCF members had a higher baseline dose of tacrolimus per kilogram of body weight (Table 2). In FCF, the TLR3-mRNA expression was negatively affected by a baseline tacrolimus dose in mg/kg (Table 4). Also, FCF graft recipients were older than donors and the TLR3-mRNA expression was negatively affected by donor age (Tables 2, 4). There were no differences in the distribution of baseline BMI (P=0.13) between UCF and FCF. In FCF, TLR3 mRNA expression was differentially associated with baseline BMI values: low BMI had a negative effect but high BMI had a positive effect (Table 4).
In UCF, our data showed no association of baseline eGFR with TLR3 mRNA expression and no association of TLR3 mRNA expression with follow-up eGFR. In this cohort, 61.1% of the recipients (P=0.024) had a baseline TLR3 mRNA expression below the median for the overall cohort of transplant recipients (Low-TLR3). Although we found no association between tacrolimus dose (in mg per kilogram of body weight) and TLR3 mRNA expression (P=0.11), there was an association between tacrolimus concentration and TLR3 mRNA expression. Regression analysis showed that the TLR3-mRNA expression in UCF was adversely affected by delayed graft function episodes, both by history (yes/no) and duration (days) (Table 4). In UCF, our analyzes of the distribution of BMI values indicated more recipients with a BMI >30 (chi-square=5.12; P=0.024). Also, we found a mild positive association of normal range baseline BMI (BMI 20–28) with TLR3-mRNA expression (Table 4). In UCF, the protein concentration in the morning urine sample was higher (P=0.025) than in FCF.
Discussion
FOLLOW-UP: IMPROVEMENT – FAVORABLE COURSE OF OBSERVATION (FCF) VS NO IMPROVEMENT – UNFAVORABLE COURSE OF OBSERVATION (UCF):
There was no difference in the baseline eGFR values between the UCF and FCF groups. The qualitative analysis showed that 59.3% of members of the baseline High-TLR3-mRNA group had higher follow-up eGFR values and were finally assigned to the FCF group. In FCF, the baseline expression of TLR3-mRNA was higher than that in the UCF group. Therefore, it should be assumed that FCF had different proportions of individual PBMC populations than UCF [31,36,37]. Around 80% of patients in the FCF group had baseline GFR in the range of grade 3. Only former members of the High-TLR3-mRNA group with moderately impaired baseline graft function (GFR grade 3b) showed improvement in renal function during the follow-up period (Figure 2). In the FCF group, we also detected patients with low baseline TLR3 mRNA expression. Some of these people could have different polymorphisms, which, apart from affecting the expression of Toll-like receptors, also reduced the intensity of PBMC stimulation. These polymorphisms tend to reduce rejection rates but increase the risk of infectious complications or autoimmunity [3,38]. However, the relative impact of patients with good graft function and possibly low TLR3-mRNA expression of unstimulated PBMCs was small. In the FCF group, in the biopsies taken before the start of the follow-up period, kidney recipients with a subsequent high TLR3-mRNA (High-TLR3 group) were distinguished by lower glomerular and small arteriolar inflammatory activity. Kidney recipients later classified as FCF were more likely to receive the more effective immunosuppression (tacrolimus+mycophenolate mofetil) at the start of the follow-up period. Bearing in mind that low expression of TLR3-mRNA (Low-TLR3-mRNA) usually coexisted with low baseline eGFR in the general cohort, we completed the analysis with a prospective assessment of changes in kidney function in the Low-TLR3-mRNA group. Tests for related observations suggested that a significant proportion of the Low-TLR3-mRNA group consisted of patients in whom renal function would not improve but would rather remain stably impaired with the possibility of slow deterioration (Figure 3).
Kidney recipients with eGFR >60 ml/min (60+) constitute a special group of patients. At the beginning of the follow-up period, eGFR distribution parameters did not differ between patients with a favorable (FCF60+) or unfavorable course (UCF60-) of follow-up. In those recipients whose kidney function improved further, TLR3-mRNA expression was higher than in the control population and was much higher than in UCF60-. In the UCF60- and TLR3-, mRNA expression was lower than in the control population [24,25,39].
Tacrolimus is the most widely used calcineurin inhibitor [19,32,40]. Under the influence of calcineurin inhibitors in macrophages, an inflammatory stimulation of TLR expression is secondarily inhibited. The effect of using calcineurin inhibitors is to increase the chance of transformation towards the anti-inflammatory phenotype [40]. Diurnal variability in tacrolimus concentrations is difficult to demonstrate with an ambulatory immunosuppression monitoring system that relies on standardized concentration measurements before the morning dose only. This is a compromise between precision, burdensome transplant supervision, and costs of care [20,41–43]. We found a positive relationship between tacrolimus dose per kg body weight and TLR3-mRNA expression in the FCF group. It should be noted that the FCF group had a lower proportion of kidney recipients with a baseline BMI >30 and higher baseline doses of tacrolimus/kg body weight. On the other hand, in the UCF group, we found a positive relationship between morning tacrolimus concentration (t0) and TLR3-mRNA expression, and a positive relationship between BMI values and TLR3-mRNA expression. In our opinion, this is an argument indicating that BMI contributed to an increase in overall inflammatory activity [37,42,44–46]. Tacrolimus and cyclosporin A are highly lipophilic and have variable bioavailability depending on many factors [36]. Although we found no differences in the baseline concentrations of cyclosporin A and tacrolimus between UCF and FCF, the effectiveness of treatment was better in the FCF group. The variability characteristics of tacrolimus concentration may be more favorable in leaner recipients [47].
Kidney recipients in the FCF group were also distinguished by fewer episodes of delayed graft function and good biological quality of the kidney associated with the young age of the donor [44].
Conclusions
In kidney transplant recipients with moderately impaired graft function, the predominance of PBMC phenotypes with high expression of TLR3-mRNA may offer a chance to improve graft function. In patients with clearly impaired graft function, low TLR3-mRNA expression may coexist with unfavorable and already completed development of the inflammatory process in the transplanted kidney. Further studies on a large patient population are needed to confirm our observations.
Limitations
The problem of viral infections was not addressed in the study. The issues of viral infections (including CMV, HSV, HCV, HBV) will be the subject of the next study, which is currently in preparation.
Figures
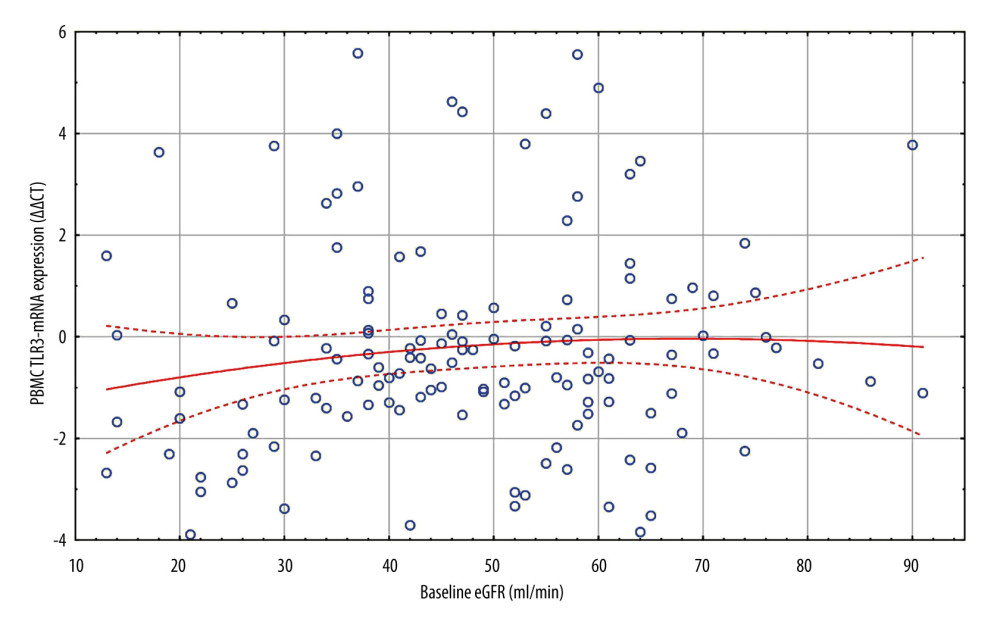 Figure 1. All patients. Scatterplot of baseline eGFR versus TLR3-mRNA expression. d0 eGFR: PBMC TLR3-mRNA (ΔΔCT): y=−0.8862+0.13*x; r=0.1059; p=0.2213. PBMC TLR3-mRNA ΔΔCT=−1.5572+0.0444*x−0.0003*x2; 0.95 Conf.int. d0 eGFR – baseline eGFR; eGFR – estimated glomerular filtration rate (ml/min); mRNA – messenger RNA; PBMC – peripheral blood mononuclear cells; TLR3 – Toll-like receptor 3; ΔΔCt – “delta,deltaCt” measure and method of expression of mRNA of Toll-like receptors. The figure was prepared using Statistica 13 PL software.
Figure 1. All patients. Scatterplot of baseline eGFR versus TLR3-mRNA expression. d0 eGFR: PBMC TLR3-mRNA (ΔΔCT): y=−0.8862+0.13*x; r=0.1059; p=0.2213. PBMC TLR3-mRNA ΔΔCT=−1.5572+0.0444*x−0.0003*x2; 0.95 Conf.int. d0 eGFR – baseline eGFR; eGFR – estimated glomerular filtration rate (ml/min); mRNA – messenger RNA; PBMC – peripheral blood mononuclear cells; TLR3 – Toll-like receptor 3; ΔΔCt – “delta,deltaCt” measure and method of expression of mRNA of Toll-like receptors. The figure was prepared using Statistica 13 PL software. 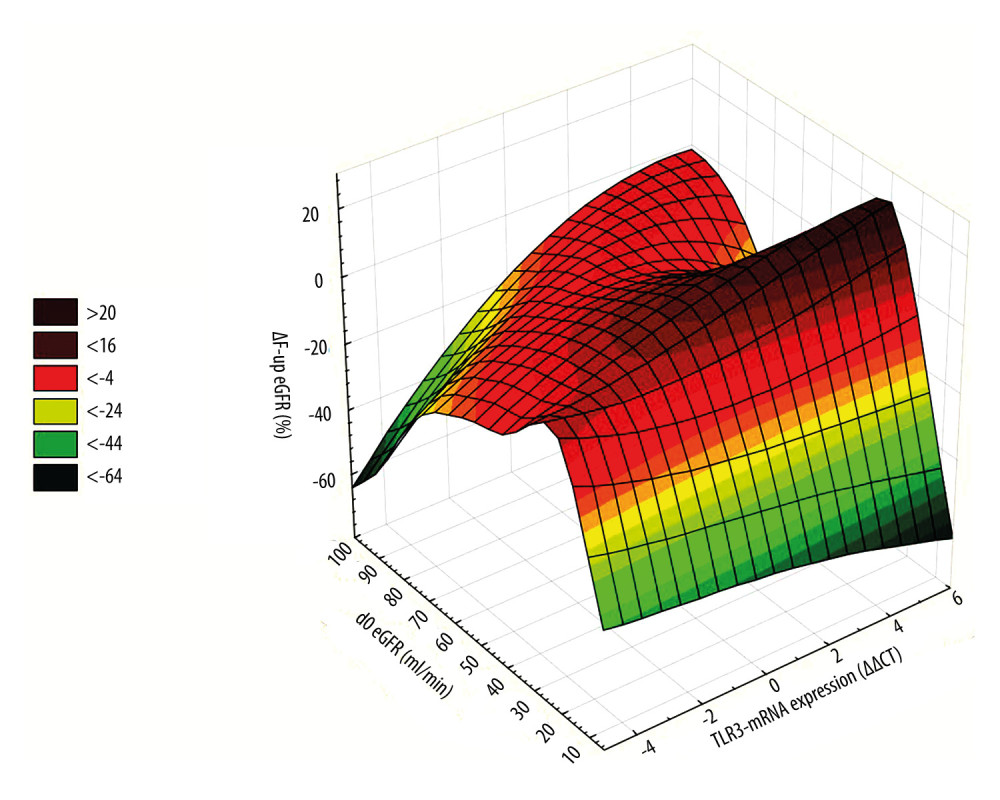 Figure 2. 3D ΔF-up eGFR area plot against TLR3-mRNA expression and baseline eGFR. ΔF-up eGFR – Distance weighted least squares smoothing. d0 eGFR – baseline eGFR; mRNA – messenger RNA; PBMC – peripheral blood mononuclear cells; TLR3 – Toll-like receptor 3; ΔΔCt – “delta,deltaCt” measure and method of expression of mRNA of Toll-like receptors; ΔF-up eGFR – relative change (%) of eGFR at the end of follow-up. The figure was prepared using Statistica 13 PL software.
Figure 2. 3D ΔF-up eGFR area plot against TLR3-mRNA expression and baseline eGFR. ΔF-up eGFR – Distance weighted least squares smoothing. d0 eGFR – baseline eGFR; mRNA – messenger RNA; PBMC – peripheral blood mononuclear cells; TLR3 – Toll-like receptor 3; ΔΔCt – “delta,deltaCt” measure and method of expression of mRNA of Toll-like receptors; ΔF-up eGFR – relative change (%) of eGFR at the end of follow-up. The figure was prepared using Statistica 13 PL software. 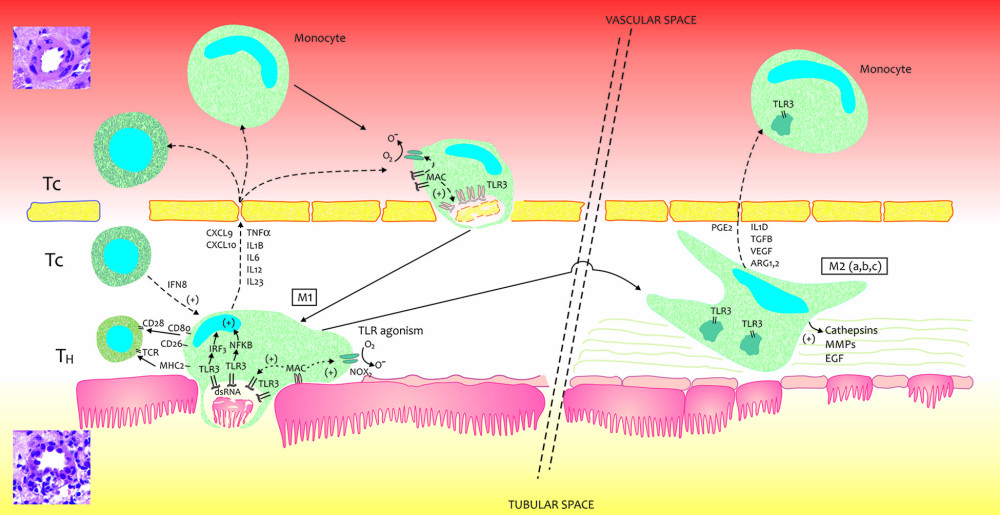 Figure 3. A very simplified graphical interpretation of the role of the Toll-like 3 receptor of peripheral blood mononuclear cells in interaction with the interstitium of a transplanted kidney. ARG – Arginase; CD – cluster of differentiation; CXCL – chemokine (C-X-C motif) ligand; EGF – Epidermal growth factor; FCF – favorable course of follow-up; IFNB – interferon beta; IL – Interleukin; IL1D – interleukin 1 soluble domain; IRF – Interferon regulatory factor; MHC – major histocompatibility complex; MMPs – Matrix metalloproteinases; NOX – NADPH oxidase; O- – oxygen radical; O2 – oxygen; TCR – T-cell receptor; TGFB – Transforming growth factor beta; TH – helper lymphocyte; TLR3- Toll-like receptor 3; TNF – Tumor necrosis factor; VEGF – vascular endothelial growth factor. The figure was prepared using Adobe Photoshop and Microsoft Paint.
Figure 3. A very simplified graphical interpretation of the role of the Toll-like 3 receptor of peripheral blood mononuclear cells in interaction with the interstitium of a transplanted kidney. ARG – Arginase; CD – cluster of differentiation; CXCL – chemokine (C-X-C motif) ligand; EGF – Epidermal growth factor; FCF – favorable course of follow-up; IFNB – interferon beta; IL – Interleukin; IL1D – interleukin 1 soluble domain; IRF – Interferon regulatory factor; MHC – major histocompatibility complex; MMPs – Matrix metalloproteinases; NOX – NADPH oxidase; O- – oxygen radical; O2 – oxygen; TCR – T-cell receptor; TGFB – Transforming growth factor beta; TH – helper lymphocyte; TLR3- Toll-like receptor 3; TNF – Tumor necrosis factor; VEGF – vascular endothelial growth factor. The figure was prepared using Adobe Photoshop and Microsoft Paint. References
1. Karimi-Googheri M, Arababadi MK, TLR3 plays significant roles against hepatitis B virus: Mol Biol Rep, 2014; 41; 3279-86
2. Yuan MM, Xu YY, Chen L, TLR3 expression correlates with apoptosis, proliferation and angiogenesis in hepatocellular carcinoma and predicts prognosis: BMC Cancer, 2015; 15; 245
3. Gosu V, Son S, Shin D, Song K-D, Insights into the dynamic nature of the dsRNA-bound TLR3 complex: Sci Rep, 2019; 9; 3652
4. Chen Y, Lin J, Zhao Y, Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses: J Zhejiang Univ Sci B, 2021; 22; 609-32
5. Liu Y, Liu T, Lei T, RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (review): Int J Mol Med, 2019; 44; 771-86
6. Cavassani KA, Ishii M, Wen H, TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events: J Exp Med, 2008; 205; 2609-21
7. Gollmann-Tepekoylu C, Graber M, Polzl L, Toll-like receptor 3 mediates ischaemia/reperfusion injury after cardiac transplantation: Eur J Cardiothorac Surg, 2020; 57; 826-35
8. Vijay K, Toll-like receptors in immunity and inflammatory diseases: Past, present, and future: Int Immunopharmacol, 2018; 59; 391-412
9. Zinngrebe J, Rieser E, Taraborrelli L, LUBAC deficiency perturbs TLR3 signaling to cause immunodeficiency and autoinflammation: J Exp Med, 2016; 213; 2671-89
10. Royer PJ, Henrio K, Pain M, TLR3 promotes MMP-9 production in primary human airway epithelial cells through Wnt/β-catenin signaling: Respir Res, 2017; 18; 208
11. Uzureau S, Coquerelle C, Vermeiren C, Apolipoproteins L control cell death triggered by TLR3/TRIF signaling in dendritic cells: Eur J Immunol, 2016; 46; 1854-66
12. Liu B, Wang X, Chen TZ, Polarization of M1 tumor associated macrophage promoted by the activation of TLR3 signal pathway: Asian Pac J Trop Med, 2016; 9; 484-88
13. Ding X, Jin S, Tong Y, TLR4 signaling induces TLR3 up-regulation in alveolar macrophages during acute lung injury: Sci Rep, 2017; 7; 34278
14. Mielcarska MB, Bossowska-Nowicka M, Toka FN, Cell surface expression of endosomal toll-like receptors – a necessity or a superfluous duplication?: Front Immunol, 2021; 11; 620972
15. Lai R, Gu M, Jiang W, Raf kinase inhibitor protein preferentially promotes TLR3-triggered signaling and inflammation: J Immunol, 2017; 198; 4086-95
16. Liang Y, Cao X, Ding Q, Hepatitis C virus NS4B induces the degradation of TRIF to inhibit TLR3-mediated interferon signaling pathway: PLoS Pathogens, 2018; 14; e1007075
17. Zmonarski SC, Banasik M, Golebiowski T, Toll-like 4 receptor (TLR4) expression on peripheral blood mononuclear cells in renal transplant recipients with pre-transplant chronic interstitial nephritis indicates patients at risk of graft deterioration: Transpl Immunol, 2020; 62; 101319
18. Zmonarski SC, Madziarska K, Golebiowski T, Can the Toll-like receptors 4 expression in peripheral blood mononuclear cells help assess the effectiveness of immunosuppression and the chance of a future good renal transplant function?: Transpl Immunol, 2019; 53; 43-50
19. Lezaic V, Naumovic R, Stanic M, Factors affecting graft function in pediatric and adult recipients of adult live donor kidney transplants: Pediatr Transplant, 2007; 11; 906-13
20. Karcz M, Kusztal M, Boratynska M, Klinger M, Very long-term survival of the transplanted kidney-characteristics of recipients: Transplant Proc, 2018; 50; 1730-32
21. Zmonarski SC, Banasik M, Gołębiowski T, Toll-like 4 receptor expression on peripheral blood mononuclear cells in renal transplant recipients can help to indicate the risk of graft deterioration in patients who experienced an episode of symptomatic cytomegalovirus infection: Transplant Proc, 2020; 52(8); 2394-402
22. Rigoni M, Torri E, Nollo G, Survival and time-to-transplantation of peritoneal dialysis versus hemodialysis for end-stage renal disease patients: Competing-risks regression model in a single Italian center experience: J Nephrol, 2017; 30; 441-47
23. Suresh MV, Dolgachev VA, Zhang B, TLR3 absence confers increased survival with improved macrophage activity against pneumonia: JCI Insight, 2019; 4; e131195
24. Meng XM, Tang PM, Li J, Lan HY, Macrophage phenotype in kidney injury and repair: Kidney Dis (Basel), 2015; 1; 138-46
25. Toki D, Zhang W, Hor KL, The role of macrophages in the development of human renal allograft fibrosis in the first year after transplantation: Am J Transplant, 2014; 14; 2126-36
26. Haas M, Chronic allograft nephropathy or interstitial fibrosis and tubular atrophy: What is in a name?: Curr Opin Nephrol Hypertens, 2014; 23; 245-50
27. Banasik M, Boratynska M, Koscielska-Kasprzak K, The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes: Transpl Immunol, 2013; 30(1); 24-29
28. Eleftheriadis T, Pissas G, Sounidaki M, Uric acid increases cellular and humoral alloimmunity in primary human peripheral blood mononuclear cells: Nephrology (Carlton), 2018; 23(7); 610-15
29. Liu H, Xiong J, He T, High uric acid-induced epithelial-mesenchymal transition of renal tubular epithelial cells via the TLR4/NF-κB signaling pathway: Am J Nephrol, 2017; 46; 333-42
30. Rogacev KS, Zawada AM, Hundsdorfer J, Immunosuppression and monocyte subsets: Nephrol Dial Transplant, 2015; 30; 143-53
31. Howell J, Sawhney R, Testro A, Cyclosporine and tacrolimus have inhibitory effects on toll-like receptor signaling after liver transplantation: Liver Transpl, 2013; 19; 1099-107
32. Qiu L, Lai X, Wang J-J, Kidney-intrinsic factors determine the severity of ischemia/reperfusion injury in a mouse model of delayed graft function: Kidney Int, 2020; 98; 1489-501
33. Andrade-Oliveira V, Campos EF, Goncalves-Primo A, TLR4 mRNA levels as tools to estimate risk for early posttransplantation kidney graft dysfunction: Transplantation, 2012; 94; 589-95
34. Zmonarski SC, Koscielska-Kasprzak K, Banasik M, Lowering of messenger ribonucleic acid toll-like receptors 2–4,9 in peripheral blood mononuclear cells in kidney allograft recipients, relationships with immunosuppressive treatment, and delayed graft function occurrence: Transplant Proc, 2016; 48; 1519-25
35. Zmonarski S, Madziarska K, Banasik M, Expression of PBMC TLR4 in renal graft recipients who experienced delayed graft function reflects dynamic balance between blood and tissue compartments and helps select a problematic patient: Transplant Proc, 2018; 50; 1744-49
36. Vanhove T, Annaert P, Kuypers DR, Clinical determinants of calcineurin inhibitor disposition: A mechanistic review: Drug Metab Rev, 2016; 48; 88-112
37. Kunz HE, Hart CR, Gries KJ, Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity: Am J Physiol Endocrinol Metab, 2021; 321; E105-21
38. Hwang YH, Ro H, Choi I, Impact of polymorphisms of TLR4/CD14 and TLR3 on acute rejection in kidney transplantation: Transplantation, 2009; 88; 699-705
39. Schnaper HW, The tubulointerstitial pathophysiology of progressive kidney disease: Adv Chronic Kidney Dis, 2017; 24; 107-16
40. Bendickova K, Tidu F, Fric J, Calcineurin-NFAT signalling in myeloid leucocytes: New prospects and pitfalls in immunosuppressive therapy: EMBO Mol Med, 2017; 9; 990-99
41. Jouve T, Noble J, Rostaing L, Malvezzi P, Tailoring tacrolimus therapy in kidney transplantation: Expert Rev Clin Pharmacol, 2018; 11; 581-88
42. Chang JY, Yu J, Chung BH, Immunosuppressant prescription pattern and trend in kidney transplantation: A multicenter study in Korea: PLoS One, 2017; 12; e0183826
43. Peeters LEJ, Andrews LM, Hesselink DA, Personalized immunosuppression in elderly renal transplant recipients: Pharmacol Res, 2018; 130; 303-7
44. Goring SM, Levy AR, Ghement I, A network meta-analysis of the efficacy of belatacept, cyclosporine and tacrolimus for immunosuppression therapy in adult renal transplant recipients: Curr Med Res Opin, 2014; 30; 1473-87
45. Jawitz OK, Raman V, Klapper J, Donor and recipient age matching in heart transplantation: analysis of the UNOS Registry: Transplant Int, 2019; 32; 1194-202
46. Hall JE, Mouton AJ, da Silva AA, Obesity, kidney dysfunction, and inflammation: interactions in hypertension: Cardiovasc Res, 2021; 117; 1859-76
47. Robert V, Manos-Sampol E, Manson T, Tacrolimus exposure in obese patients: And a case-control study in kidney transplantation: Ther Drug Monit, 2021; 43; 229-37
Figures
 Figure 1. All patients. Scatterplot of baseline eGFR versus TLR3-mRNA expression. d0 eGFR: PBMC TLR3-mRNA (ΔΔCT): y=−0.8862+0.13*x; r=0.1059; p=0.2213. PBMC TLR3-mRNA ΔΔCT=−1.5572+0.0444*x−0.0003*x2; 0.95 Conf.int. d0 eGFR – baseline eGFR; eGFR – estimated glomerular filtration rate (ml/min); mRNA – messenger RNA; PBMC – peripheral blood mononuclear cells; TLR3 – Toll-like receptor 3; ΔΔCt – “delta,deltaCt” measure and method of expression of mRNA of Toll-like receptors. The figure was prepared using Statistica 13 PL software.
Figure 1. All patients. Scatterplot of baseline eGFR versus TLR3-mRNA expression. d0 eGFR: PBMC TLR3-mRNA (ΔΔCT): y=−0.8862+0.13*x; r=0.1059; p=0.2213. PBMC TLR3-mRNA ΔΔCT=−1.5572+0.0444*x−0.0003*x2; 0.95 Conf.int. d0 eGFR – baseline eGFR; eGFR – estimated glomerular filtration rate (ml/min); mRNA – messenger RNA; PBMC – peripheral blood mononuclear cells; TLR3 – Toll-like receptor 3; ΔΔCt – “delta,deltaCt” measure and method of expression of mRNA of Toll-like receptors. The figure was prepared using Statistica 13 PL software. Figure 2. 3D ΔF-up eGFR area plot against TLR3-mRNA expression and baseline eGFR. ΔF-up eGFR – Distance weighted least squares smoothing. d0 eGFR – baseline eGFR; mRNA – messenger RNA; PBMC – peripheral blood mononuclear cells; TLR3 – Toll-like receptor 3; ΔΔCt – “delta,deltaCt” measure and method of expression of mRNA of Toll-like receptors; ΔF-up eGFR – relative change (%) of eGFR at the end of follow-up. The figure was prepared using Statistica 13 PL software.
Figure 2. 3D ΔF-up eGFR area plot against TLR3-mRNA expression and baseline eGFR. ΔF-up eGFR – Distance weighted least squares smoothing. d0 eGFR – baseline eGFR; mRNA – messenger RNA; PBMC – peripheral blood mononuclear cells; TLR3 – Toll-like receptor 3; ΔΔCt – “delta,deltaCt” measure and method of expression of mRNA of Toll-like receptors; ΔF-up eGFR – relative change (%) of eGFR at the end of follow-up. The figure was prepared using Statistica 13 PL software. Figure 3. A very simplified graphical interpretation of the role of the Toll-like 3 receptor of peripheral blood mononuclear cells in interaction with the interstitium of a transplanted kidney. ARG – Arginase; CD – cluster of differentiation; CXCL – chemokine (C-X-C motif) ligand; EGF – Epidermal growth factor; FCF – favorable course of follow-up; IFNB – interferon beta; IL – Interleukin; IL1D – interleukin 1 soluble domain; IRF – Interferon regulatory factor; MHC – major histocompatibility complex; MMPs – Matrix metalloproteinases; NOX – NADPH oxidase; O- – oxygen radical; O2 – oxygen; TCR – T-cell receptor; TGFB – Transforming growth factor beta; TH – helper lymphocyte; TLR3- Toll-like receptor 3; TNF – Tumor necrosis factor; VEGF – vascular endothelial growth factor. The figure was prepared using Adobe Photoshop and Microsoft Paint.
Figure 3. A very simplified graphical interpretation of the role of the Toll-like 3 receptor of peripheral blood mononuclear cells in interaction with the interstitium of a transplanted kidney. ARG – Arginase; CD – cluster of differentiation; CXCL – chemokine (C-X-C motif) ligand; EGF – Epidermal growth factor; FCF – favorable course of follow-up; IFNB – interferon beta; IL – Interleukin; IL1D – interleukin 1 soluble domain; IRF – Interferon regulatory factor; MHC – major histocompatibility complex; MMPs – Matrix metalloproteinases; NOX – NADPH oxidase; O- – oxygen radical; O2 – oxygen; TCR – T-cell receptor; TGFB – Transforming growth factor beta; TH – helper lymphocyte; TLR3- Toll-like receptor 3; TNF – Tumor necrosis factor; VEGF – vascular endothelial growth factor. The figure was prepared using Adobe Photoshop and Microsoft Paint. Tables
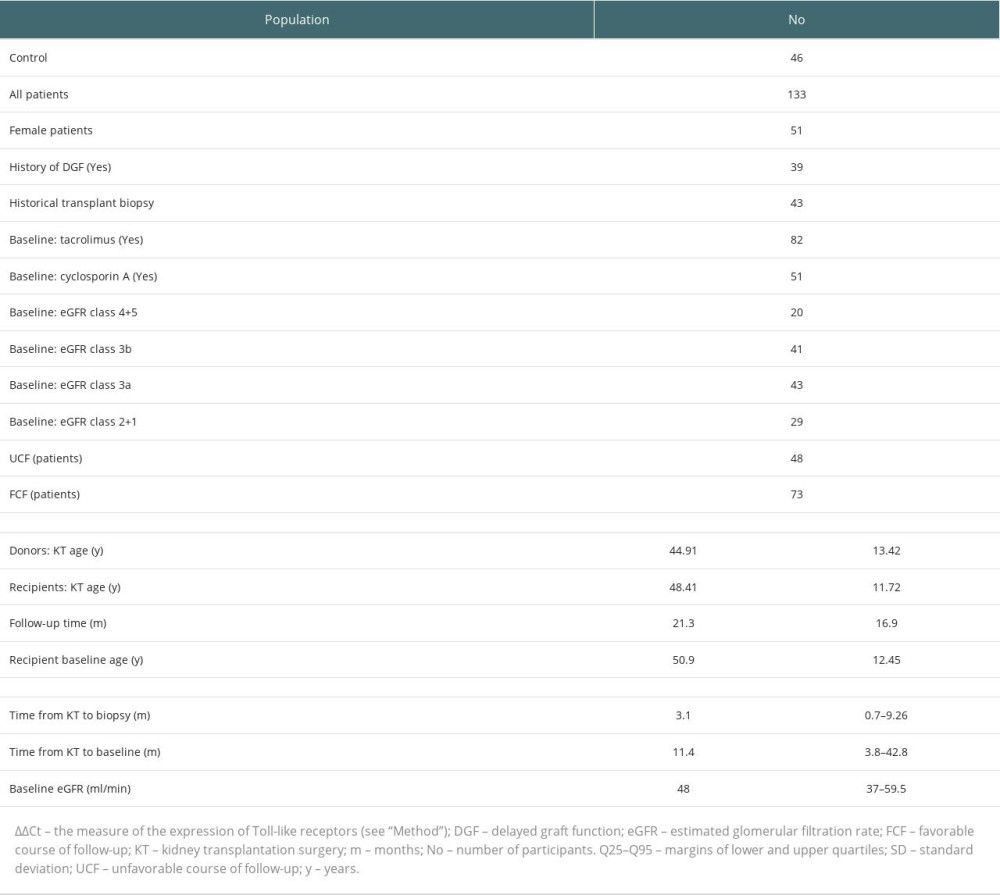 Table 1. Study population.
Table 1. Study population.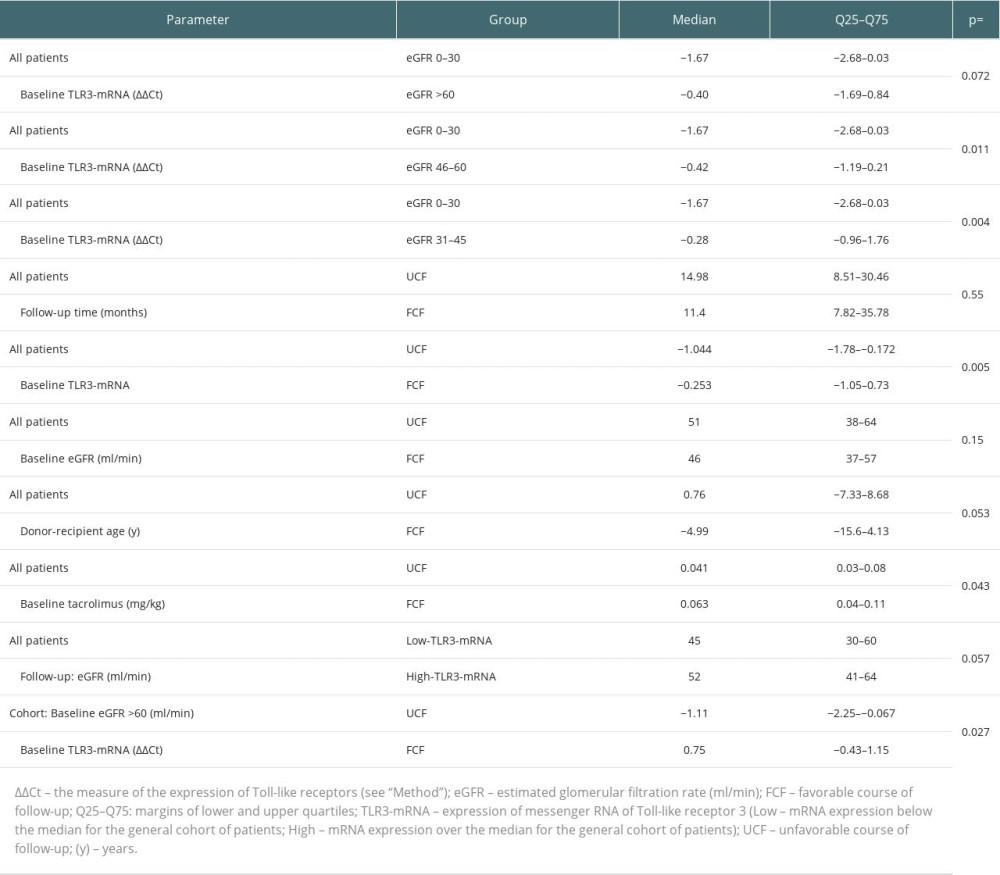 Table 2. Comparison of unrelated continuous variables.
Table 2. Comparison of unrelated continuous variables.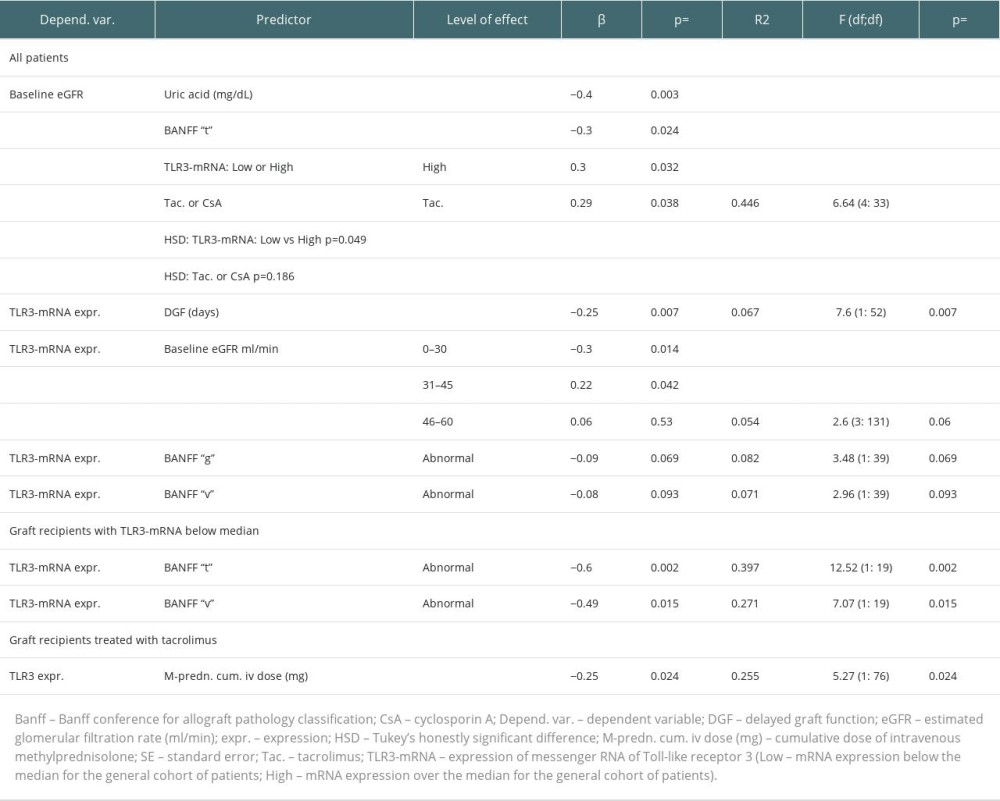 Table 3. Regression analysis of baseline data.
Table 3. Regression analysis of baseline data.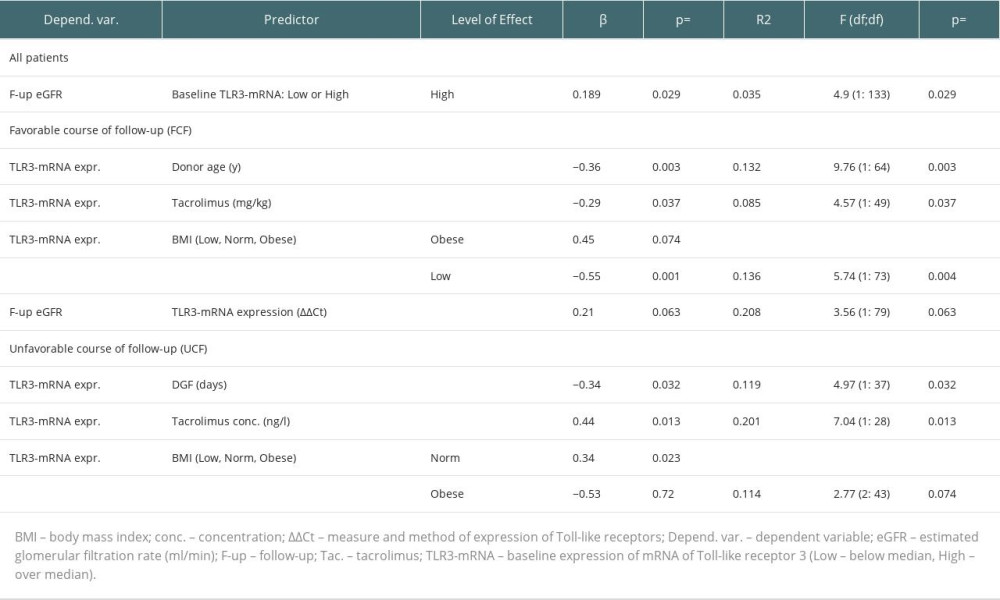 Table 4. Follow-up, regression analysis of UCF and FCF groups.
Table 4. Follow-up, regression analysis of UCF and FCF groups.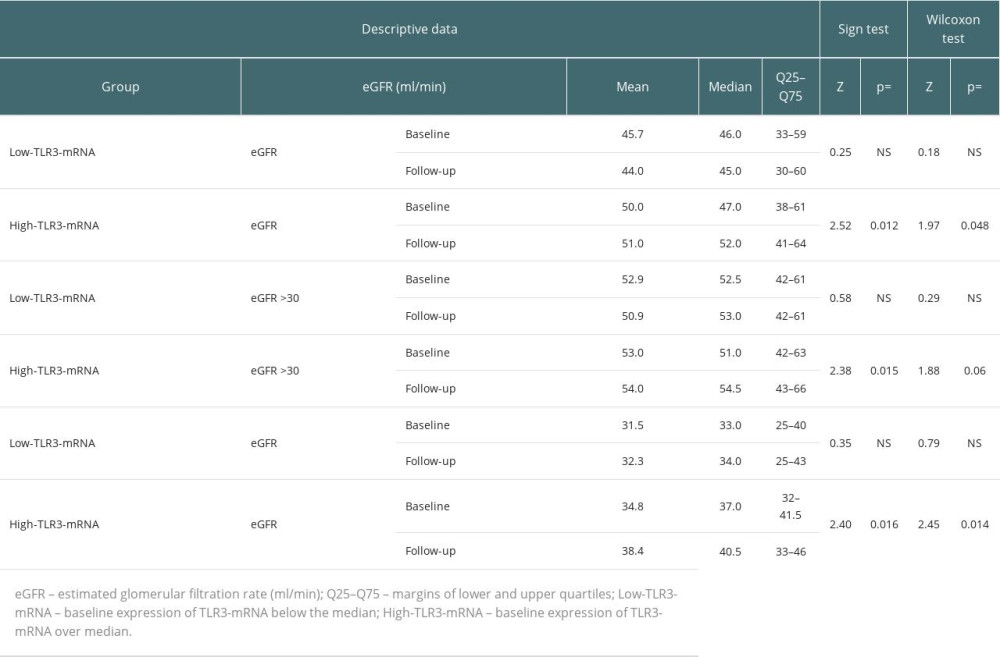 Table 5. Baseline vs follow-up assessment of the variability of related observations.
Table 5. Baseline vs follow-up assessment of the variability of related observations. Table 1. Study population.
Table 1. Study population. Table 2. Comparison of unrelated continuous variables.
Table 2. Comparison of unrelated continuous variables. Table 3. Regression analysis of baseline data.
Table 3. Regression analysis of baseline data. Table 4. Follow-up, regression analysis of UCF and FCF groups.
Table 4. Follow-up, regression analysis of UCF and FCF groups. Table 5. Baseline vs follow-up assessment of the variability of related observations.
Table 5. Baseline vs follow-up assessment of the variability of related observations. In Press
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








