05 July 2022: Original Paper
Cardiac and Pulmonary Histopathology in Baboons Following Genetically-Engineered Pig Orthotopic Heart Transplantation
Silvio H. Litovsky1BDE, Jeremy B. Foote2BCDE, Abhijit Jagdale3BE, Gregory Walcott4BE, Hayato Iwase3BDE, Mohamed H. Bikhet3BDE, Takayuki Yamamoto3BE, Christophe Hansen-Estruch3BE, Mohamed B. Ezzelarab5BE, David Ayares6AE, Waldemar F. Carlo7BE, Leslie A. Rhodes7BE, Jack H. Crawford8BE, Santiago Borasino8BE, Robert J. Dabal8BE, Luz A. Padilla8BE, Hidetaka Hara3BE, David K.C. Cooper3ABDEFG*, David C. Cleveland8ABDEFGDOI: 10.12659/AOT.935338
Ann Transplant 2022; 27:e935338
Abstract
BACKGROUND: Although improving, survival after pig orthotopic heart transplantation (OHTx) in baboons has been mixed and largely poor. The causes for the high incidence of early failure remain uncertain.
MATERIAL AND METHODS: We have carried out pig OHTx in 4 baboons. Two died or were euthanized within hours, and 2 survived for 3 and 8 months, respectively. There was evidence of a significant ‘cytokine storm’ in the immediate post-OHTx period with the elevations in IL-6 correlating closely with the final outcome.
RESULTS: All 4 baboons demonstrated features suggestive of respiratory dysfunction, including increased airway resistance, hypoxia, and tachypnea. Histopathological observations of pulmonary infiltration by neutrophils and, notably, eosinophils within vessels and in the perivascular and peribronchiolar space, with minimal cardiac pathology, suggested a role for early lung acute inflammation. In one, features suggestive of transfusion-related acute lung injury were present. The 2 longer-term survivors died of (i) a cardiac dysrhythmia with cellular infiltration around the conducting tissue (at 3 months), and (ii) mixed cellular and antibody-mediated rejection (at 8 months).
CONCLUSIONS: These initial findings indicate a potential role of acute lung injury early after OHTx. If this response can be prevented, increased survival may result, providing an opportunity to evaluate the factors affecting long-term survival.
Keywords: Xenotransplantation, Heart Transplantation, lungs, pig, Baboon, Animals, Antibodies, Heart Transplantation, Lung, Papio, Swine, Transplantation, Heterologous
Background
Complex congenital heart disease in neonates and infants, particularly single ventricle pathology, continues to be associated with significant mortality and morbidity despite advances in surgical and medical techniques [1]. Orthotopic heart transplantation (OHTx) might offer the best prospect of long-term survival of the patient, but the availability of hearts of an appropriate size from deceased human donors remains very limited. For infants with inoperable congenital heart disease or failed single ventricle palliation, bridging by mechanical cardiac assist devices is associated with poor results [2].
We suggest that, with recent advances in the xenotransplantation of genetically-engineered pig hearts [3,4] and kidneys [5–8], bridging with a pig heart may be more successful. However, heart function after pig OHTx in nonhuman primates (NHPs) has been mixed and largely poor [3,9–11]. Early graft failure does
To date, we have a very limited experience of pig OHTx in baboons (n=4), but we have made some observations that may throw light on the factors leading to early and late graft failure. We have carried out 4 such transplants using 2 types of genetically-engineered pigs (Table 1). The anesthetic management, surgical technique, postoperative care, and immunosuppressive and adjunctive regimens have been described previously [12]. Cardiopulmonary bypass (CPB) involved priming with blood, but no further blood transfusions were given. There were no technical complications during OHTx. The immunosuppressive therapeutic regimen of T and B cell depletion, followed by an anti-CD40mAb-based maintenance regimen, was identical to that used successfully in baboons receiving pig kidney grafts [6]. Two baboons died within a few hours, and 2 survived for several months (Table 1).
Although some aspects of this experience have been discussed elsewhere [12,13], we believe the histopathological findings are worthy of comment as they suggest a major role for lung inflammation as a factor in the early deaths, and draw attention to the possible role of eosinophils and transfusion-related acute lung injury (TRALI) which have not been reported previously.
Material and Methods
All animal care was in accordance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Results
CLINICAL COURSES:
Once CPB was discontinued, all baboons demonstrated tachycardia and hypertension which, in the longer-term survivors, persisted for several weeks (Table 1). In the hours following weaning from the ventilator, all 4 baboons demonstrated features of respiratory dysfunction (e.g., tachypnea, reduced pO2 and O2 saturation (Table 1). Cytokine and histopathology observations suggest a critical role for acute pulmonary dysfunction in the early deaths of 2 baboons, but not in the 2 that survived for 3 and 8 months, respectively.
Cardioplegic arrest was with University of Wisconsin (UW) solution in one case (B217), and the pig heart did not function well following discontinuation of CPB. In the other 3 experiments, in which Del Nido cardioplegia was used, immediate cardiac function (as judged by hemodynamics, echocardiography, and need for inotropic support, was good) [12]. In one case (B37367), the baboon was probably extubated too quickly, and this was followed by hypoxia leading to dysrhythmias.
ACUTE INFLAMMATORY CYTOKINE LEVELS DURING THE PERI-OPERATIVE PERIOD AND POST TRANSPLANTATION:
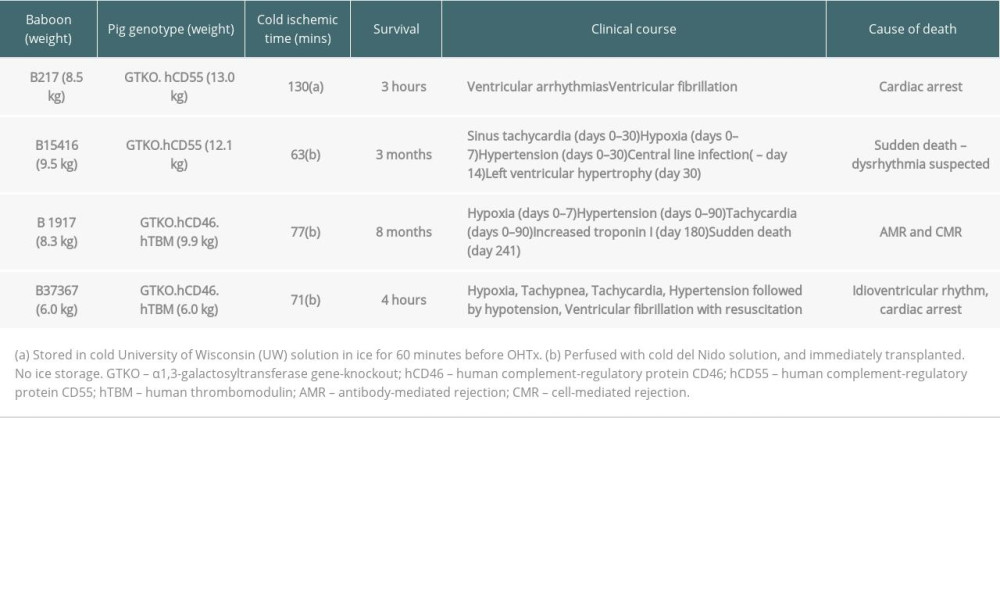
In the current report, we summarize previously reported details of the acute inflammatory cytokine response after transplantation (Figure 1) [13]. The most important observation was the much greater increase in IL-6 that occurred in the 2 baboons that died within 4 hours from cardiopulmonary failure (Figure 1). In the 2 longer-surviving baboons, there was a delayed and temporary increase in IL-8 after approximately 2 weeks, but this was associated with features suggesting an infectious complication, and had normalized by 4 weeks.
HISTOPATHOLOGY OF PIG HEARTS AND BABOON LUNGS:
After death of the baboon, the pig heart and baboon lungs were removed and fixed in10% neutral buffered formalin saline for at least 24 h. After gross examination, several blocks of both ventricles, interventricular septum, and all lobes of the lungs were cut, dehydrated, embedded in paraffin, sectioned (5uM), and stained with H&E (and Masson’s trichrome). Histopathological features were independently evaluated by 2 board-certified pathologists (SL and JF) prior to confirmation of the findings reported below.
SHORT-TERM SURVIVORS:
B217 (survived 3 hours): The heart revealed multiple foci of hemorrhage and myocardial necrosis, most prominently within the left ventricle (Figure 2A, 2B). There was no evidence of fibrin thrombi or neutrophilic infiltrate, suggesting that the histopathologic lesions were secondary to ischemia-reperfusion injury. The lungs did not exhibit significant cellular infiltration in the alveoli or bronchi, but significant numbers of intravascular neutrophils were present within the medium-to-small caliber branches of the pulmonary arteries (Figure 2C, 2D), as well as pulmonary edema (not shown).
B37367 (survival 4 hours): The pig heart showed subendocardial hemorrhage and contraction band necrosis, typical of ischemia-reperfusion injury (not shown). The baboon lungs (Figure 3A–3D) showed inflammation affecting both the airways and the vasculature. The airways showed prominent inflammation that extended to the adjacent tissue (Figure 3A), and involved the respiratory epithelium (Figure 3B). The pleomorphic infiltrate included a significant number of eosinophils (Figure 3B). The vasculature was heavily inflamed (Figure 3C), and the inflammation extended to the perivascular and vascular tissue (Figure 3D). As with the airways, the inflammatory response included a significant number of eosinophils that were also seen in the lumen. Of interest, a complete blood count obtained in the immediate postoperative period showed leukopenia (2.7K) with neutropenia (1.89K), and lymphocytopenia (0.24K), but normal eosinophil count (0.24K), with a relative eosinophilia (9%).
LONGER-TERM SURVIVORS:
B15416 (survival 3 months): The baboon remained clinically well, and died suddenly. At necropsy, the pig heart was grossly enlarged compared to the day of transplantation (increase in weight from 30 g to 150 g). Histopathological features of inflammation and fibrosis were present within the myocardial interstitium and around the Purkinje fibers (Figure 4A), suggesting a dysrhythmia as the cause of death, similar to that reported after cardiac allotransplantation [14]. The inflammatory infiltrate included a significant number of eosinophils (Figure 4B). There were chronic and acute/subacute changes in the coronary arteries, including intimal hyperplasia (not shown) and mature fibrin thrombi (Figure 4C). A number of arteries showed endothelial activation (Figure 4D), suggesting the possibility of antibody-mediated rejection. The lungs exhibited mild-to-moderate fibrosis within the alveolar septa, around the pulmonary arteries, and at the pleural surfaces (not shown). Within the alveolar interstitium and surrounding branches of the pulmonary artery, there was a mild lymphocytic infiltrate (not shown). Small foci of calcification in medium-caliber arteries were also seen (not shown).
B1917 (survival 8 months): The baboon did well for 7 months, at which time there was an increase in troponin I (not shown). At necropsy, the pig heart was grossly enlarged (increase in weight from 40 g to 264 g). Histologic examination of the coronary arteries showed acute and subacute thrombi (not shown), often in a background of layers of intimal hyperplasia (Figure 5A). The veins, perivasculature, and myocardial interstitium exhibited inflammation that included a high number of eosinophils (Figure 5B and 5C respectively). Within the ventricular septum and at the base of the heart there were areas of remote (dark blue on trichrome) and more recent fibrosis (light blue on trichrome, Figure 5D). Immunohistochemistry confirmed antibody-mediated rejection, based on IgM (Figure 6A) and C4d (Figure 6B) localization to the endothelium capillaries. Within the interventricular septum there was extensive fibrosis and lymphoid aggregates that by immunohistochemistry corresponded to significant numbers of CD3+T cells (Figure 6C) and IgM+ CD20low B cells (Figure 6D, and data not shown). The baboon lungs were unremarkable (not shown). Of interest, a complete blood count obtained prior to euthanasia showed a normal white blood cell count (8.3K), with 71% neutrophils, 12% lymphocytes, and 12% eosinophils, suggesting the vascular eosinophilic infiltrate was accompanied by systemic eosinophilia.
Discussion
For this study, we purposely selected relatively small baboons in an effort to mimic clinical OHTx in infants. Our limited experience to date suggests that respiratory dysfunction, secondary to an acute inflammatory state, may be a key factor in the failure of pig hearts early after OHTx in NHPs. Both CPB and organ xenotransplantation result in an inflammatory response, associated with a major release of cytokines (reviewed in 13). Both pig kidney and heterotopic heart transplantation are associated with a fall in plasma free triiodothyronine (fT3) [6,15], another marker of inflammation, which also occurs after CPB [16–18]. We have measured increases in several cytokines/chemokines, e.g., IL-6, TNF, and MCP-1, which increase post-xenotransplantation [13,19].
In the baboon that received a pig heart protected by University of Wisconsin (UW) cardioplegic solution, we believe that primary graft dysfunction contributed to early death. In the other 3 baboons, in which cardioplegic arrest was with Del Nido solution, early graft function was good. However, when our results of early function are compared with those of others in which a system of continuous graft perfusion was utilized to minimize ischemia [3,4], they suggest that continuous perfusion is preferable unless the myocardial ischemic period is very short. However, graft function can be preserved using a crystalloid cardioplegic solution if the ischemic period is short.
In the 2 baboons that died within hours we noted marked elevations in IL-6, suggesting an initial cytokine storm. IL-6 is induced by CPB [20], specifically associated with post-CPB left ventricular wall motion abnormalities and myocardial ischemic episodes. Both IL-6 and IL-8 can act as potent chemoattractants capable of eosinophil and neutrophil recruitment [21–23]. IL-6 plays a major role in the inflammatory responses associated with both CPB [24–26] and organ xenotransplantation [15].
In a murine model, Kumar et al [27] showed that C-reactive protein enhances antibody- mediated transfusion-related acute lung injury, and it is well established that IL-6 induces the hepatic synthesis of C-reactive protein. Although we have included tocilizumab therapy (to reduce the effect of the inflammatory cytokine, IL-6) in our pig kidney xenotransplantation model for several years [6,28], we did not include it in the present study because our more recent research indicates that it prevented IL-6 binding to
Pulmonary histologic evaluation of these 2 cases revealed striking pulmonary findings. B37367 showed prominent inflammation in the airways, including epithelium and adjacent areas, and in the pulmonary medium- and small-sized arteries (including in the media), and perivascular areas. The pleomorphic infiltrate showed a significant eosinophilic component. To our knowledge, this has not been described in solid organ xenotransplants. Pancreatic islet xenografts in mice showed strong eosinophilic infiltration (4+), while eosinophils were ‘rare’ in allografts [30]. Peripheral eosinophilia is associated with poor outcome post-lung transplantation [31].
B37367 showed a relative eosinophilia during the immediate postoperative period when the pulmonary and hemodynamic status deteriorated, leading to its demise. Conditions like early-phase SARS-CoV-2 are typically accompanied by eosinophilia [32], and the relative eosinophilia in this baboon points toward a mechanism that promotes eosinophil recruitment to several organs and tissues.
In contrast, B217 displayed an accumulation of neutrophils in the pulmonary microvasculature, and pulmonary edema, mimicking the histopathology findings of transfusion-related acute lung injury (TRALI), a condition in which a blood transfusion triggers the activation of neutrophils in the lung microvasculature [33,34]. (All of the baboons received blood in the CPB machine.) In B217, it seems likely that the cause of death was primarily associated with ischemia-reperfusion injury, possibly resulting from inadequate myocardial protection, but there may have been a contribution from compromised gas exchange and the high inotropic therapy that was necessary.
In our 2 longer-surviving baboons, the evidence for an inflammatory response in the immediate post-transplant period was more modest, correlating with the fact that these baboons survived this period. Nevertheless, B15416 still showed some features of inflammatory lung injury when it suddenly died 3 months’ later.
Both baboons showed evidence of antibody-mediated rejection with endothelial activation, intimal hyperplasia, and thrombi in the coronary arteries. B1917 (the longest survivor) had prominent eosinophilic infiltration around both arteries and veins, suggesting that eosinophils played a role in the thrombotic diathesis. The eosinophilic infiltrate was accompanied by eosinophilia. In B15416 the inflammation centered on the Purkinje fibers, without evidence of myocardial damage from coronary thrombosis, supporting the conclusion that death resulted from a sudden dysrhythmia. In contrast, B1917 showed foci of remote and recent myocardial infarction, associated with a rise in troponin, suggesting that graft arteriopathy had been developing.
In summary, the involvement of eosinophils and the similarities of histologic findings and TRALI require investigation.
In both cases, the increase in size and weight of the hearts had been extensive. In B1917, this was in part associated with the presence of severe rejection, and in both baboons it was probably also associated with the rapid growth of the pig organ, as reported by us and others in relation to both kidney and heart xenotransplantation [3,5,6,35]. The transplantation of hearts from pigs genetically-engineered by growth hormone receptor gene-knockout, as proposed by Hinrichs and his colleagues [36,37], may resolve this problem [38]. Rapamycin has been included in our immunosuppressive regimen for many years, and may have reduced growth of the pig hearts [37].
Conclusions
If the problem of the initial inflammatory response to pig OHTx could be overcome, this might facilitate more consistent long-term survival of the pig heart graft.
Figures
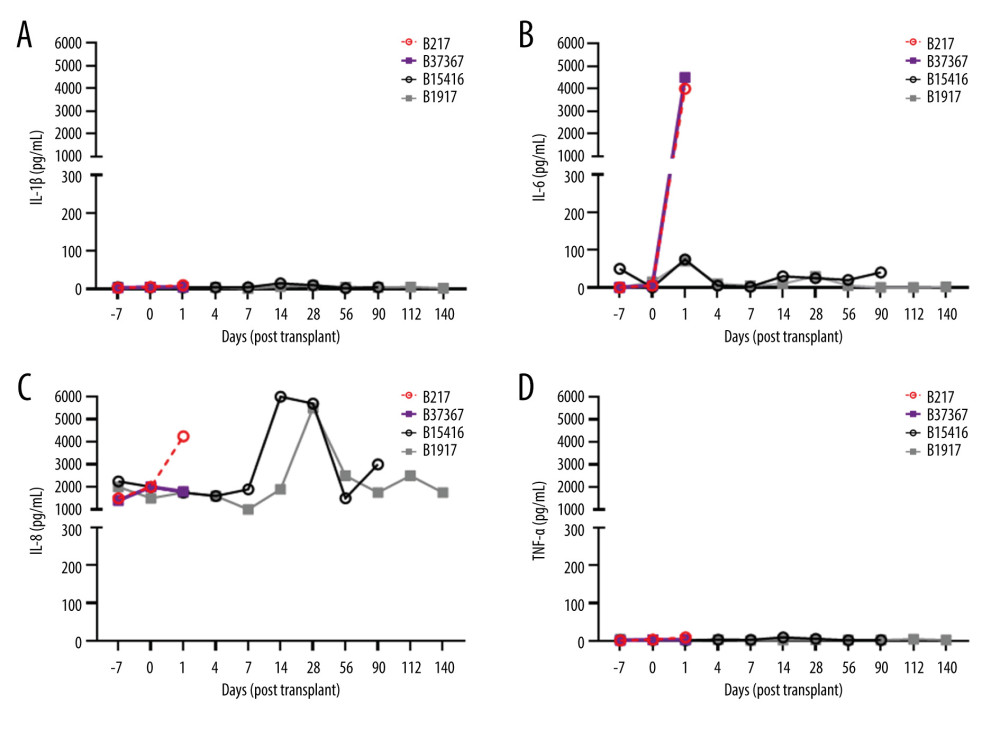 Figure 1. Elevations in pro-inflammatory cytokines after pig OHTx in baboons (n=4)(A) IL-1β, (B) IL-6, (C) IL-8, (D) TNFα were measured at different time-points by Luminex multianalyte ELISA.
Figure 1. Elevations in pro-inflammatory cytokines after pig OHTx in baboons (n=4)(A) IL-1β, (B) IL-6, (C) IL-8, (D) TNFα were measured at different time-points by Luminex multianalyte ELISA. 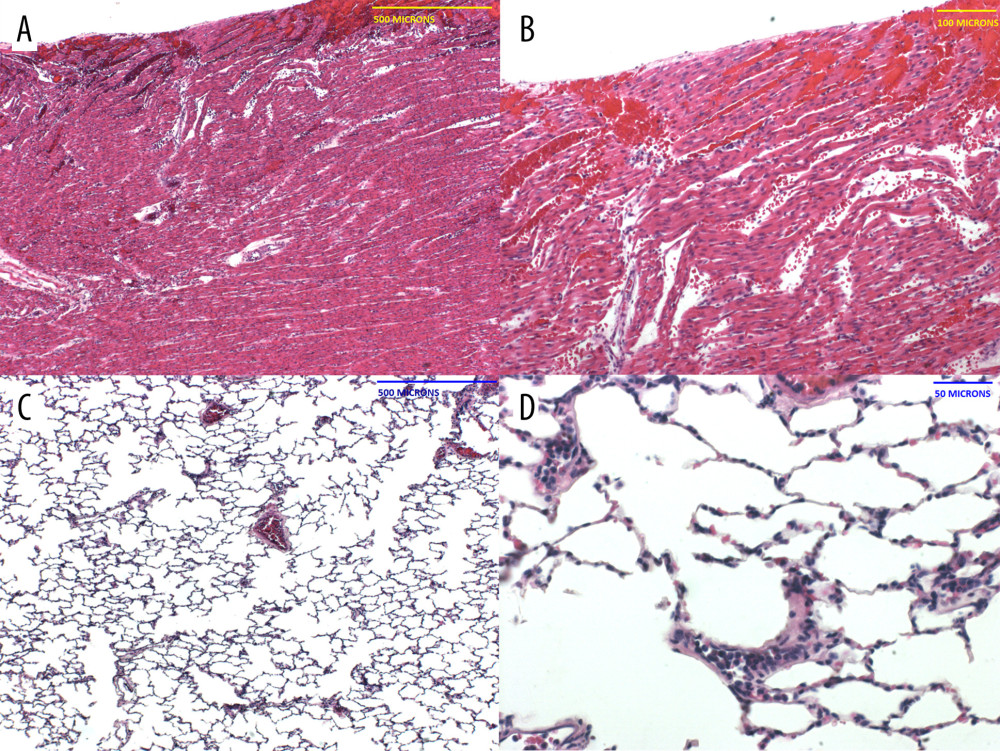 Figure 2. Histopathology of the pig heart (A, B) and baboon lungs (C, D) in B217 at necropsy(A, B) The heart showed multifocal foci of hemorrhage and myocardial necrosis, particularly within the left ventricle, suggestive of ischemia-reperfusion injury. (C, D) The lungs exhibited minimal lesions in airways and alveoli, but showed clusters of neutrophils in small vessels, and pulmonary edema (not shown).
Figure 2. Histopathology of the pig heart (A, B) and baboon lungs (C, D) in B217 at necropsy(A, B) The heart showed multifocal foci of hemorrhage and myocardial necrosis, particularly within the left ventricle, suggestive of ischemia-reperfusion injury. (C, D) The lungs exhibited minimal lesions in airways and alveoli, but showed clusters of neutrophils in small vessels, and pulmonary edema (not shown). 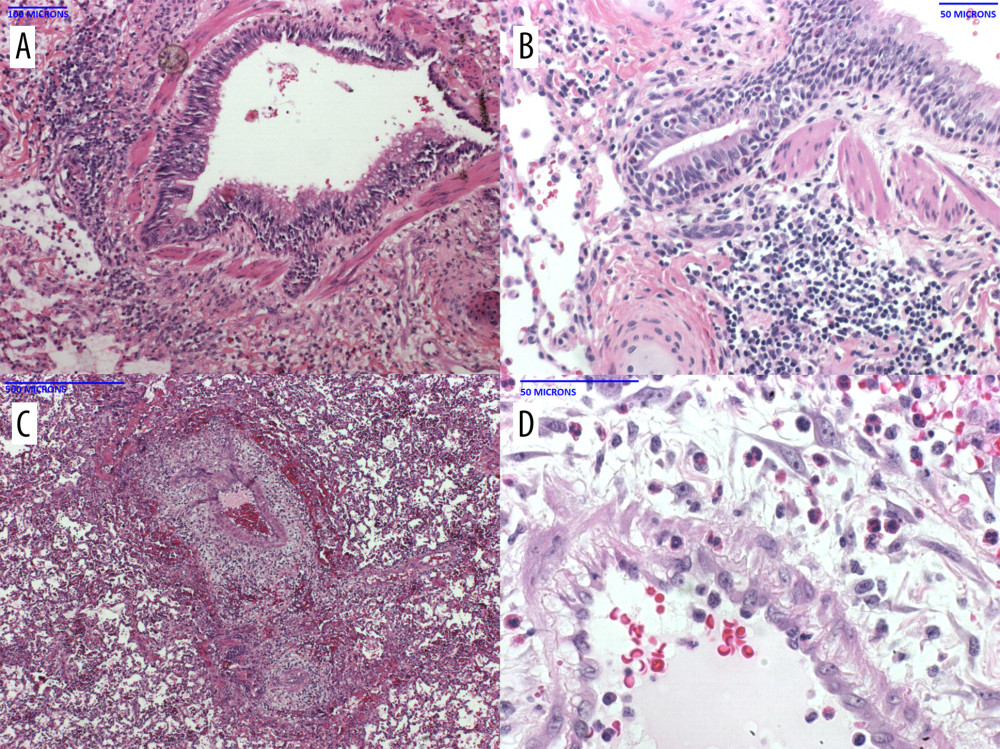 Figure 3. Histopathology of the baboon lungs (A–D) in B37367 at necropsyThe lungs showed diffuse prominent airway inflammation (A) that included the airway epithelium (B). The infiltrate contained a high number of eosinophils. The arteries were also inflamed, as was the perivascular tissue (C). Eosinophils were seen in the perivascular tissue and vascular media (D).
Figure 3. Histopathology of the baboon lungs (A–D) in B37367 at necropsyThe lungs showed diffuse prominent airway inflammation (A) that included the airway epithelium (B). The infiltrate contained a high number of eosinophils. The arteries were also inflamed, as was the perivascular tissue (C). Eosinophils were seen in the perivascular tissue and vascular media (D). 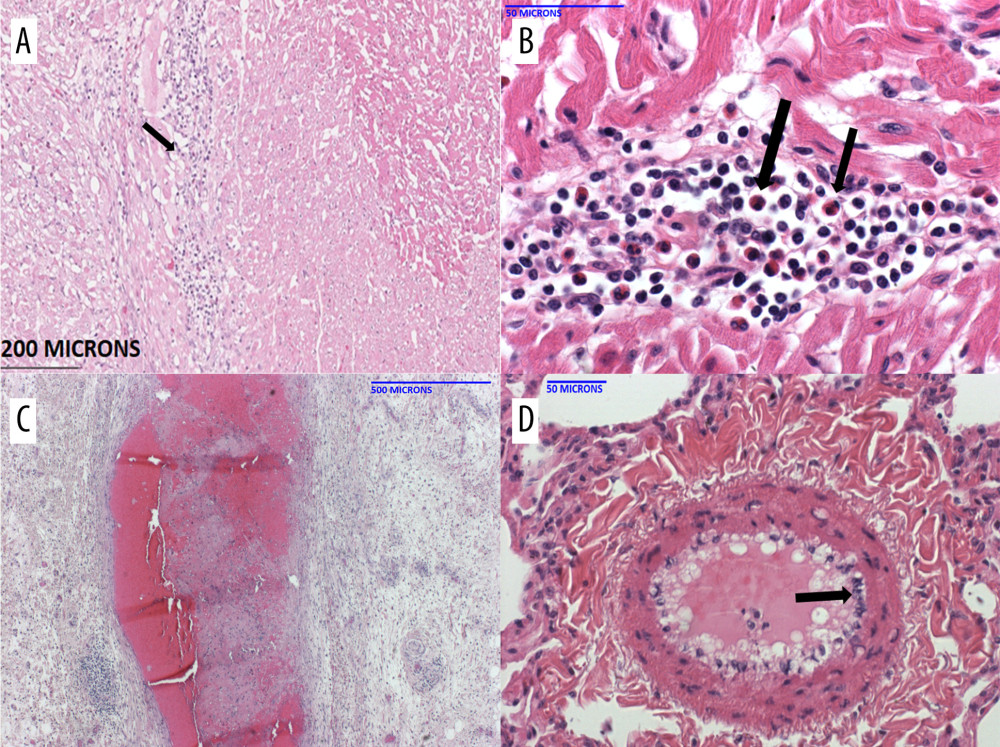 Figure 4. Histopathology of the pig heart (A–D) in B15416 at necropsy(A) The heart showed mild inflammation and fibrosis within the myocardial interstitium, and around the Purkinje fibers. Higher magnification showed a fair number of eosinophils in the infiltrate (B). The coronary arteries (C) exhibited significant mature fibrin thrombi, resulting in narrow intravascular lumens. Within the lungs (and heart, not shown), there were multiple medium-caliber arteries with endothelial activation (D, arrow).
Figure 4. Histopathology of the pig heart (A–D) in B15416 at necropsy(A) The heart showed mild inflammation and fibrosis within the myocardial interstitium, and around the Purkinje fibers. Higher magnification showed a fair number of eosinophils in the infiltrate (B). The coronary arteries (C) exhibited significant mature fibrin thrombi, resulting in narrow intravascular lumens. Within the lungs (and heart, not shown), there were multiple medium-caliber arteries with endothelial activation (D, arrow). ![Histopathology of the pig heart (A–D) in B1917 at necropsy(A) Coronary artery with layers of intimal hyperplasia, suggesting prior episodes of luminal thrombosis followed by organization. (B) Veins showed subendothelial (and perivascular) inflammation that contained a mixed inflammatory infiltrate that included eosinophils (arrow). (C) The myocardial interstitium showed areas of mixed inflammation including eosinophils (arrows). (D) The myocardial interstitium showed evidence of remote scarring (dark blue; white arrow) and more recent scarring (light blue; black arrow) [trichrome stain].](https://jours.isi-science.com/imageXml.php?i=anntransplant-27-e935338-g005.jpg&idArt=935338&w=1000) Figure 5. Histopathology of the pig heart (A–D) in B1917 at necropsy(A) Coronary artery with layers of intimal hyperplasia, suggesting prior episodes of luminal thrombosis followed by organization. (B) Veins showed subendothelial (and perivascular) inflammation that contained a mixed inflammatory infiltrate that included eosinophils (arrow). (C) The myocardial interstitium showed areas of mixed inflammation including eosinophils (arrows). (D) The myocardial interstitium showed evidence of remote scarring (dark blue; white arrow) and more recent scarring (light blue; black arrow) [trichrome stain].
Figure 5. Histopathology of the pig heart (A–D) in B1917 at necropsy(A) Coronary artery with layers of intimal hyperplasia, suggesting prior episodes of luminal thrombosis followed by organization. (B) Veins showed subendothelial (and perivascular) inflammation that contained a mixed inflammatory infiltrate that included eosinophils (arrow). (C) The myocardial interstitium showed areas of mixed inflammation including eosinophils (arrows). (D) The myocardial interstitium showed evidence of remote scarring (dark blue; white arrow) and more recent scarring (light blue; black arrow) [trichrome stain]. 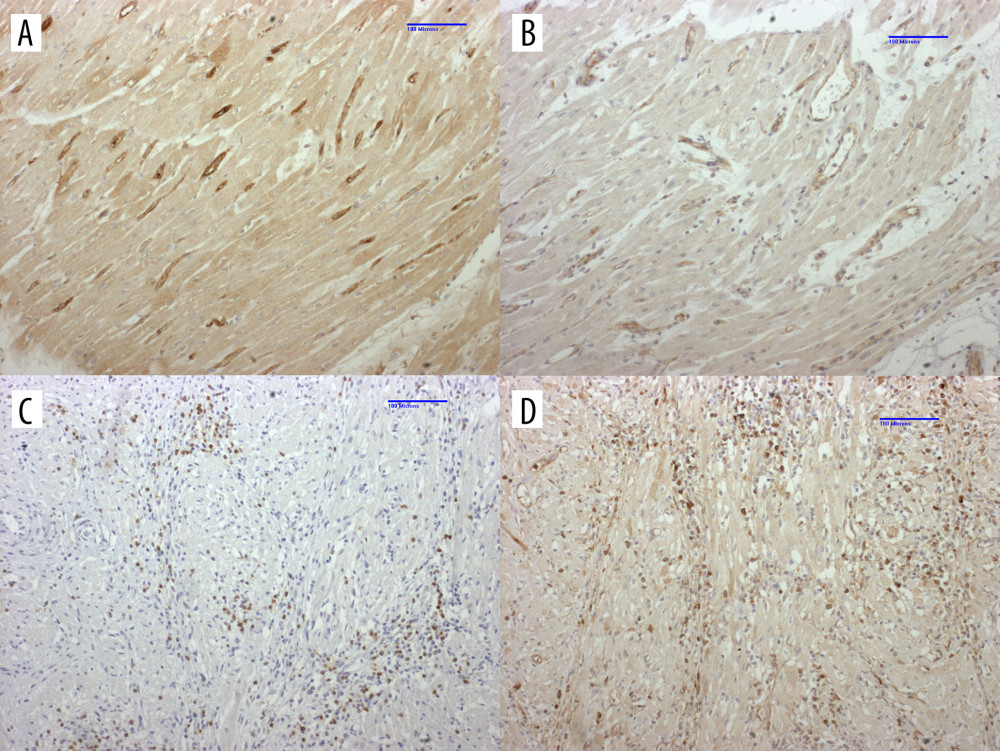 Figure 6. Immunohistochemistry of heart in B1917 was consistent with acute antibody-mediated and chronic cell-mediated rejectionLeft ventricle with significant IgM (A) and C4d (B) staining of interstitial capillary endothelium is consistent with antibody-mediated rejection. Interventricular septum displays significant numbers of CD3+ T cells (C) and IgM+ B cells (D) that is consistent with cell-mediated rejection
Figure 6. Immunohistochemistry of heart in B1917 was consistent with acute antibody-mediated and chronic cell-mediated rejectionLeft ventricle with significant IgM (A) and C4d (B) staining of interstitial capillary endothelium is consistent with antibody-mediated rejection. Interventricular septum displays significant numbers of CD3+ T cells (C) and IgM+ B cells (D) that is consistent with cell-mediated rejection References
1. Cleveland D, Banks CA, Hara H, The case for cardiac xenotransplantation in neonates: is now the time to reconsider xenotransplantation for hypoplastic left heart syndrome?: Pediatr Cardiol, 2019; 40; 437-44
2. Peng DM, Koehl DA, Cantor RS, Outcomes of children with congenital heart disease implanted with ventricular assist devices: An analysis of the Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs): J Heart Lung Transplant, 2019; 38; 420-30
3. Langin M, Mayr T, Reichart B, Consistent success in life-supporting porcine cardiac xenotransplantation: Nature, 2018; 564; 430-33
4. Reichart B, Langin M, Radan R, Pig-to-nonhuman primate heart transplantation: The final step toward clinical xenotransplantation?: J Heart Lung Transplant, 2020; 39; 751-57
5. Iwase H, Liu H, Wijkstrom M, Pig kidney graft survival in a baboon for 136 days: Longest life-supporting organ graft survival to date: Xenotransplantation, 2015; 22; 302-9
6. Iwase H, Hara H, Ezzelarab M, Immunological and physiologic observations in baboons with life-supporting genetically-engineered pig kidney grafts: Xenotransplantation, 2017; 24; e12293
7. Adams AB, Kim SC, Martens GR, Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival: Ann Surg, 2018; 268; 564-73
8. Kim SC, Mathews DV, Breeden CP, Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion: Am J Transplant, 2019; 19; 2174-85
9. Byrne GW, Du Z, Sun YW, Changes in cardiac gene expression after pig-to-primate orthotopic xenotransplantation: Xenotransplantation, 2011; 18; 14-27
10. Byrne GW, McGregor CG, Cardiac xenotransplantation: Progress and challenges: Curr Opin Organ Transplant, 2012; 17; 148-54
11. DiChiacchio L, Singh AK, Lewis B, Early experience with preclinical perioperative cardiac xenograft dysfunction in a single program: Ann Thorac Surg, 2020; 109; 1357-61
12. Cleveland DC, Jagdale A, Carlo WF, The genetically engineered heart as a bridge to allotransplantation in infants: Just around the corner?: Ann Thorac Surg, 2021 [Online ahead of print]
13. Thompson C, Jagdale A, Walcott G, The potential detrimental role of inflammation in pig orthotopic heart xenotransplantation: Xenotransplantation, 2021; 28; e12687
14. Knight CS, Tallaj JA, Rayburn BK, Bradycardia and syncope as a presentation of cardiac allograft rejection involving the conducting system: Cardiovasc Path, 2010; 19; 117-20
15. Iwase H, Ekser B, Hara H, Thyroid hormone: Relevance to xenotransplantation: Xenotransplantation, 2016; 23; 293-99
16. Novitzky D, Human PA, Cooper DKC, Inotropic effect of triiodothyronine following myocardial ischaemia and cardiopulmonary bypass: An experimental study in pigs: Ann Thorac Surg, 1988; 45; 50-55
17. Novitzky D, Cooper DKC, Swanepoel A, Inotropic effect of triiodothyronine (T3) in low cardiac output following cardioplegic arrest and cardiopulmonary bypass: An initial experience in patients undergoing open heart surgery: Eur J Cardiothorac Surg, 1989; 3; 140-45
18. Priest JR, Slee A, Olson AK, Trioodothyronine supplementation and cytokines during cardiopulmonary bypass in infants and children: J Thorac Cardiovasc Surg, 2012; 144; 938-43
19. Ezzelarab MB, Ekser B, Azimzadeh A, Systemic inflammation in xenograft recipients precedes activation of coagulation: Xenotransplantation, 2015; 22; 32-47
20. Hill GE, Cardiopulmonary bypass-induced inflammation: Is it important?: J Cardiothorac Vasc Anesth, 1998; 12(2 Suppl); 21-25
21. Erger RA, Casale TB, Interleukin-8 is a potent mediator of eosinophil chemotaxis through endothelium and epithelium: Am J Physiol, 1995; 268(1 Pt1); L117-22
22. Oliveria SH, Faccioli LH, Cunha FQ, Ferreira SH, Participation of interleukin-5 and interleukin-8 in the eosinophil migration induced by a large volume of saline: Int Arch Allergy Immunol, 1996; 111; 244-52
23. Fielding CA, McLoughlin RM, McLeod L, IL-6 regulates neutrophil trafficking during acute inflammation via STAT3: J Immunol, 2008; 181; 2189-95
24. Song M, Kellum JA, Interleukin-6: Crit Care Med, 2005; 53; S463-65
25. Halter J, Steinberg J, Fink G, Evidence of systemic cytokine release in patients undergoing cardiopulmonary bypass: J Extra Corpor Technol, 2005; 27; 272-77
26. Allan CK, Newburger JW, McGrath E, The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass: Anesth Analg, 2010; 111; 1244-51
27. Kapur R, Kim M, Shanmugabhavananthan S, C-reactive protein enhances murine antibody-mediated transfusion-related acute lung injury: Blood, 2017; 126; 2747-51
28. Iwase H, Liu H, Wijkstrom M, Pig kidney graft survival in a baboon for 136 days: Longest life-supporting organ graft survival to date: Xenotransplantation, 2015; 22; 302-9
29. Zhang G, Iwase H, Wang L, Is interleukin-6 receptor blockade (tocilizumab) beneficial or detrimental to pig-to-baboon organ xenotransplantation?: Am J Transplant, 2020; 20; 999-1013
30. Smith RM, Mandel TE, Pancreatic islet xenotransplantation: The potential for tolerance induction: Immunol Today, 2000; 21; 42-48
31. Kaes J, Van der Borgt E, Vanstaple A, Peripheral blood eosinophilia is associated with poor outcomes post-lung transplantation: Cells, 2020; 9; 2516
32. Fraissé M, Logre E, Mentec H, Eosinophilia in critically ill COVID-19 patients: A French monocenter retrospective study: Crit Care, 2020; 24; 635
33. Danielson C, Benjamin RJ, Mangano MM, Pulmonary pathology of rapidly fatal transfusion-related acute lung injury reveals minimal evidence of diffuse alveolar damage or alveolar granulocyte infiltration: Transfusion, 2008; 48; 2401-8
34. Bux J, Sachs UJH, The pathogenesis of transfusion-related acute lung injury (TRALI): Br J Hematol, 2007; 136; 788-99
35. Iwase H, Klein E, Cooper DKC, Physiologic aspects of pig kidney transplantation in nonhuman primates: Comp Med, 2018; 68; 332-40
36. Hinrichs A, Kessler B, Kurome M, Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver: Mol Metab, 2018; 11; 113-28
37. Iwase H, Ball S, Adams WT, Growth hormone receptor knockout: Relevance to xenotransplantation: Xenotransplantation, 2020; 28; e12652
38. Goerlich CE, Griffith B, Hanna P, The growth of xenotransplanted hearts can be reduced with growth hormone receptor knockout pig donors: J Thorac Cardiovasc Surg, 2021 [Online ahead of print]
Figures
 Figure 1. Elevations in pro-inflammatory cytokines after pig OHTx in baboons (n=4)(A) IL-1β, (B) IL-6, (C) IL-8, (D) TNFα were measured at different time-points by Luminex multianalyte ELISA.
Figure 1. Elevations in pro-inflammatory cytokines after pig OHTx in baboons (n=4)(A) IL-1β, (B) IL-6, (C) IL-8, (D) TNFα were measured at different time-points by Luminex multianalyte ELISA. Figure 2. Histopathology of the pig heart (A, B) and baboon lungs (C, D) in B217 at necropsy(A, B) The heart showed multifocal foci of hemorrhage and myocardial necrosis, particularly within the left ventricle, suggestive of ischemia-reperfusion injury. (C, D) The lungs exhibited minimal lesions in airways and alveoli, but showed clusters of neutrophils in small vessels, and pulmonary edema (not shown).
Figure 2. Histopathology of the pig heart (A, B) and baboon lungs (C, D) in B217 at necropsy(A, B) The heart showed multifocal foci of hemorrhage and myocardial necrosis, particularly within the left ventricle, suggestive of ischemia-reperfusion injury. (C, D) The lungs exhibited minimal lesions in airways and alveoli, but showed clusters of neutrophils in small vessels, and pulmonary edema (not shown). Figure 3. Histopathology of the baboon lungs (A–D) in B37367 at necropsyThe lungs showed diffuse prominent airway inflammation (A) that included the airway epithelium (B). The infiltrate contained a high number of eosinophils. The arteries were also inflamed, as was the perivascular tissue (C). Eosinophils were seen in the perivascular tissue and vascular media (D).
Figure 3. Histopathology of the baboon lungs (A–D) in B37367 at necropsyThe lungs showed diffuse prominent airway inflammation (A) that included the airway epithelium (B). The infiltrate contained a high number of eosinophils. The arteries were also inflamed, as was the perivascular tissue (C). Eosinophils were seen in the perivascular tissue and vascular media (D). Figure 4. Histopathology of the pig heart (A–D) in B15416 at necropsy(A) The heart showed mild inflammation and fibrosis within the myocardial interstitium, and around the Purkinje fibers. Higher magnification showed a fair number of eosinophils in the infiltrate (B). The coronary arteries (C) exhibited significant mature fibrin thrombi, resulting in narrow intravascular lumens. Within the lungs (and heart, not shown), there were multiple medium-caliber arteries with endothelial activation (D, arrow).
Figure 4. Histopathology of the pig heart (A–D) in B15416 at necropsy(A) The heart showed mild inflammation and fibrosis within the myocardial interstitium, and around the Purkinje fibers. Higher magnification showed a fair number of eosinophils in the infiltrate (B). The coronary arteries (C) exhibited significant mature fibrin thrombi, resulting in narrow intravascular lumens. Within the lungs (and heart, not shown), there were multiple medium-caliber arteries with endothelial activation (D, arrow).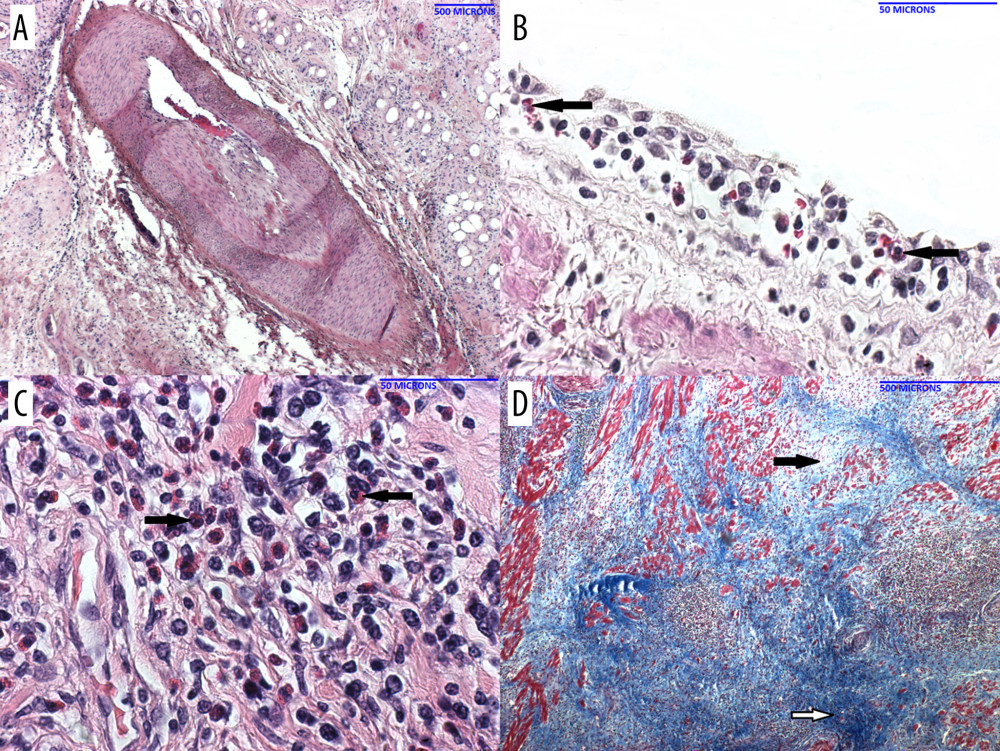 Figure 5. Histopathology of the pig heart (A–D) in B1917 at necropsy(A) Coronary artery with layers of intimal hyperplasia, suggesting prior episodes of luminal thrombosis followed by organization. (B) Veins showed subendothelial (and perivascular) inflammation that contained a mixed inflammatory infiltrate that included eosinophils (arrow). (C) The myocardial interstitium showed areas of mixed inflammation including eosinophils (arrows). (D) The myocardial interstitium showed evidence of remote scarring (dark blue; white arrow) and more recent scarring (light blue; black arrow) [trichrome stain].
Figure 5. Histopathology of the pig heart (A–D) in B1917 at necropsy(A) Coronary artery with layers of intimal hyperplasia, suggesting prior episodes of luminal thrombosis followed by organization. (B) Veins showed subendothelial (and perivascular) inflammation that contained a mixed inflammatory infiltrate that included eosinophils (arrow). (C) The myocardial interstitium showed areas of mixed inflammation including eosinophils (arrows). (D) The myocardial interstitium showed evidence of remote scarring (dark blue; white arrow) and more recent scarring (light blue; black arrow) [trichrome stain]. Figure 6. Immunohistochemistry of heart in B1917 was consistent with acute antibody-mediated and chronic cell-mediated rejectionLeft ventricle with significant IgM (A) and C4d (B) staining of interstitial capillary endothelium is consistent with antibody-mediated rejection. Interventricular septum displays significant numbers of CD3+ T cells (C) and IgM+ B cells (D) that is consistent with cell-mediated rejection
Figure 6. Immunohistochemistry of heart in B1917 was consistent with acute antibody-mediated and chronic cell-mediated rejectionLeft ventricle with significant IgM (A) and C4d (B) staining of interstitial capillary endothelium is consistent with antibody-mediated rejection. Interventricular septum displays significant numbers of CD3+ T cells (C) and IgM+ B cells (D) that is consistent with cell-mediated rejection In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860