30 April 2024: Original Paper
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of Renal Transplant Patients
Zhenwei Jiang1BCDEF, Caomei Xu1CE, Huan Hou2C, Xuping Yang1D, Minyan Qian1B, Lian Zuo1B, Peipei Wang3B, Qing Qian1D, Yan Jiang1B, Nan Hu1AG*DOI: 10.12659/AOT.943282
Ann Transplant 2024; 29:e943282
Abstract
BACKGROUND: This study aimed to investigate the incidence of post-transplant diabetes mellitus (PTDM) in renal transplant (RT) patients at our center and to explore new risk factors for PTDM.
MATERIAL AND METHODS: This retrospective study included RT patients from 2010 to 2022. Clinic data on RT patients were obtained from hospital electronic medical records. CYP3A5*3, POR*28, ABCB1 (3435 C>T), and ABCB1 (1236 C>T) were genotyped in RT patients. The associations between age, BMI, concentration of tacrolimus (TAC), polymorphism of genes, antibiotics (eg, penicillins, cephalosporins, oxazolidinones, quinolones), numbers and days of antibiotic use, and PTDM were analyzed.
RESULTS: In this study, 409 patients with RT were included. The cumulative incidence of PTDM in the first year after RT was 9.05%. The numbers and days of antibiotic use in PTDM patients were significantly higher than those in non-PTDM patients. Multivariate logistic regression analysis identified age (OR=1.047, P=0.014), body mass index (BMI) (OR=1.178, P=0.007), dose-adjusted trough concentration of TAC (TAC C₀/D) at 7 days after RT (OR=1.159, P=0.042), trough concentration of TAC (TAC C₀) at 28 days after RT (OR=1.094, P=0.042), and levofloxacin (OR=5.975, P=0.003) as independent risk factors for PTDM.
CONCLUSIONS: In addition to age, BMI, and TAC concentration after RT, antibiotic use may be a novel factor affecting PTDM. The use of antibiotics may influence the development of PTDM.
Keywords: Tacrolimus, Diabetes Mellitus, Antibiotic 88617, Polymorphism, Genetic
Introduction
Renal transplantation (RT) has been the most efficient treatment for end-stage renal disease. Post-transplant diabetes mellitus (PTDM) is a common metabolic complication after RT, which can lead to graft rejection, dysfunction, or even graft loss, and increases the risk of infection and cardiovascular diseases. After acute and chronic rejection, it is the second most important factor affecting the long-term survival rate of transplant patients. Therefore, it is particularly important to identify the risk factors for PTDM, especially controllable factors, to reduce the risk of PTDM and improve the quality of life of RT patients. In recent years, many retrospective clinical studies have found risk factors affecting PTDM, including race, age, family history of diabetes mellitus, body mass index (BMI), preexisting diabetes risk, and immunosuppressants, but the results are inconsistent [1,2].
Tacrolimus (TAC) is the most widely used calcineurin inhibitor in transplant patients and is often used to prevent rejection reactions after organ transplantation [3]. Long-term use of TAC can cause metabolic disorders, which is considered the major risk factor for PTDM, and 33.6% of nondiabetic solid organ transplant patients receiving TAC developed PTDM or impaired fasting blood glucose (IFBG) within 6 months after transplantation [2]. TAC is predominantly metabolized by
Long-term use of immunosuppressive agents after RT can lead to immunosuppression and increase the risk of opportunistic infections. Infection occurs in 3–53% of patients after solid organ transplantation, which seriously affects survival of the transplanted organ [7]. Therefore, the use of antibiotics is inevitable. However, long-term exposure to certain antibiotics (eg, cephalosporin, macrolide, fluoroquinolones, and penicillin) can increase the risk of diabetes [8,9]. A longer duration of antibiotic use was associated with a higher risk of diabetes, and the association between antibiotic use and diabetes risk was unlikely to be modified by age, family history of diabetes, obesity, or lifestyle factors [10]. In addition, antibiotics can disrupt gut microbiota homeostasis. As a key regulator of a variety of metabolic pathways, the gut microbiota plays an important role in maintaining intestinal physiology and homeostasis and regulating many metabolic functions [11]. The influence of gut microbiota on diabetes has been confirmed, including its involvement in the regulation of blood glucose and insulin sensitivity [12]. It has been reported that antibiotics aggravate TAC-induced glucose metabolism disorders in mice by disrupting the gut microbiota [11]. Antibiotics may play a direct or indirect role in the development of PTDM.
To explore the incidence and risk factors of PTDM, we retrospectively analyzed the clinical data of 409 RT patients who received TAC as an immunosuppressant and used antibiotics in the perioperative period.
Material and Methods
STUDY POPULATION:
We retrospectively analyzed the clinical data of RT patients receiving TAC, mycophenolate acid, and a glucocorticoid triple immunosuppression regimen. All patients received renal transplantation on the same day or the next day after hospitalization. Clinical data of RT patients from January 2010 to January 2022 were collected from the Third Affiliated Hospital of Soochow University records. Eligibility criteria were: age ≥18 years, no multiple organ transplantation, and identical immunosuppressive regimen including TAC. Exclusion criteria were: followed up less than 1 year, missing data, with a known history of pretransplant diabetes, death within the first year after RT, graft loss, switched off TAC or exposure to cyclosporine within 1 year after RT, and treated with Wuzhi capsule (a traditional Chinese medicine) or diltiazem during hospitalization. Ethics approval was obtained from the Ethics Committee of the Third Affiliated Hospital of Soochow University.
DIAGNOSTIC CRITERIA:
Based on recent International Consensus Guidelines, the cases of PTDM were identified using the American Diabetes Association (ADA) criteria for the diagnosis of diabetes, requiring at least 2 of the following: symptoms of diabetes and FBG >7 mmol/L on more than 1 occasion, random plasma glucose (RPG) ≥11.1 mmol/L, 2-h blood glucose ≥11.1 mmol/L during an oral glucose tolerance test (OGTT), or HbA1c ≥6.5%. PTDM was diagnosed at least 1.5 months after the transplantation to exclude the influence of other factors, such as surgical stress after transplantation [13].
IMMUNOSUPPRESSIVE REGIMEN:
All RT patients were initially given the standard triple therapy of TAC, mycophenolic acid, and glucocorticoids following transplantation. Mycophenolic acid was administered within 12 h before RT, with mycophenolate mofetil 500–1000 mg/day or mycophenolate sodium 360–720 mg/day twice daily. Corticosteroids were started at a dose of 500 mg/day of intravenous administration of methylprednisolone during the transplant surgery and were reduced to 250 mg/day on the 2nd or 3rd day after surgery. Thereafter, prednisone tablets were given at 40 mg/day and gradually reduced to a maintenance dose (10–20 mg/day). The initial TAC dosage was usually 0.07–0.15 mg/kg/day. The first dose was given on the 1st to 3rd day after renal transplantation. Subsequently, the dose of TAC was adjusted to maintain the TAC C0 at 10–15 ng/ml for 1 month, 8–15 ng/ml for 1 to 3 months, 5–12 ng/ml for 3 to 12 months, and 5–10 ng/ml after 12 months [14].
Whole blood (2–3 ml) was obtained from patients just before the morning dose, and the whole blood C0 of TAC was determined by enzyme-amplified immunoassay (EMIT) (Viva-E, SIEMENS, Germany).
GENOTYPING:
DNA was extracted from peripheral blood samples using AXYGEN’s AxyPrep Blood Genomic DNA Extraction Kit. CYP3A5*3, POR*28, ABCB1 (3435 C>T), and ABCB1 (1236 C>T) were genotyped by polymerase chain reaction-restriction fragment length polymorphism PCR-RFLP) [15].
ANALYSIS OF ANTIBIOTIC USE:
All antibiotics used orally or intravenously in RT patients during hospitalization were included in this study, except skin creams and mouthwash. The days of antibiotic use were calculated according to the date indicated in the prescription. Number of antibiotics used was defined as the number of specific antibiotics used per patient. Finally, penicillins, second-generation cephalosporins, third-generation cephalosporins, carbapenems, oxazolidinones, quinolones, sulfonamides, glycopeptides, polymyxins, tetracyclines, aminoglycosides, and macrolides were included in this study.
STATISTICAL ANALYSIS:
All descriptive and logistic regression analyses were conducted using SPSS 25.0 software. Categorical variables are expressed as numbers (%), and continuous variables with a normal distribution are expressed as the means±standard deviations (SD). Continuous variables with a skewed distribution are presented as the medians with interquartile range (IQR). For normally or approximately normally distributed variables, group comparisons were made using the
Results
INCIDENCE OF PTDM AND BASELINE CHARACTERISTICS OF PATIENTS:
According to the inclusion and exclusion criteria, 409 patients were included in this retrospective study, including 269 males (65.7%) and 140 females (34.2%). Patients were categorized by their diabetes status into the PTDM and non-PTDM groups. In total, 37 patients (9.05%) were diagnosed with PTDM within 1 year after RT. The median time of PTDM diagnosis was 68.00 (IQR: 56.50–79.50) days. Among them, 30 (81.1%) cases were diagnosed within 1.5–3 months after RT, 3 (8.1%) cases were diagnosed within 3–6 months, and 4 (10.8%) cases were diagnosed within 6–9 months. The FBG in the non-PTDM group was 5.09±0.71 mmol/L and was 6.92±2.49 mmol/L in the PTDM group after RT (
Table 1 presents the baseline characteristics of the PTDM group and non-PTDM group. The mean age of PTDM patients was significantly older than that of non-PTDM patients (P<0.001). Compared to non-PTDM patients, patients with PTDM tended to have a higher BMI (P<0.001). There were no significant differences in FBG between patients in the PTDM and non-PTDM groups before transplantation. For traditional risk factors, such as HBV and HCV, there were no significant differences in HBV and HCV between patients in the PTDM and non-PTDM groups.
:
We compared the C0 and C0/D of TAC at different time points between the PTDM group and the non-PTDM group within 1 year after RT, and the results are shown in Figure 1. The C0 and C0/D of TAC in PTDM patients were higher than those in non-PTDM patients. There were significant differences of TAC C0 between the PTDM group and non-PTDM group at 7, 14, and 28 days, and 6 months after RT (8.37±4.76 vs 6.80±4.08, 8.96±4.22 vs 7.72±3.41, 8.96±1.91 vs 7.94±2.67, and 8.21±2.30 vs 6.93±1.82 ng/ml, respectively). Meanwhile, the level of TAC C0/D in the PTDM group was significantly higher than non-PTDM group at 7 days and 6 months (1.57±0.84 vs 1.31±0.69, and 3.59±1.52 vs 2.32±1.41 ng/ml, respectively).
EFFECTS OF GENE POLYMORPHISMS ON TAC BLOOD CONCENTRATION AND PTDM:
We evaluated the influence of CYP3A5*3, ABCB1 (3435 C>T), ABCB1 (1236 C>T), and POR*28 polymorphisms on TAC C0 and C0/D within the first month after RT. Among them, the CYP3A5*3 allele had a significant impact on the TAC concentration. The TAC C0 and C0/D in patients with the CYP3A5*3/*3 genotype were the highest at 7, 14, 21, and 28 days after RT, followed by the patients with CYP3A5*1/*3 genotype and CYP3A5*1/*1 (Figure 2). However, there were no effects of ABCB1 (3435 C>T), ABCB1 (1236 C>T), and POR*28 polymorphisms on TAC C0 and C0/D.
The chi-square test was used to analyze the influence of CYP3A5*3, ABCB1 (3435 C>T), ABCB1 (1236 C>T), and POR*28 polymorphisms on PTDM. However, there was no significant difference in the occurrence of PTDM among patients with different genotypes (Table 2).
INFLUENCE OF ANTIBIOTIC USE ON PTDM:
We summarized the use of antibiotics in RT patients during hospitalization (Supplementary Table 1). All patients were treated with at least 2 antibiotics, with an average of 4.4±1.7 types of antibiotics used per patient. The most commonly used antibiotic was piperacillin-tazobactam (n=229), followed by imipenem-cilastatin (n=156), linezolid (n=139), moxifloxacin (n=100), ceftriaxone (n=63), cefodizime (n=39), ceftazidime (n=35), and cefotiam (n=31).
The PTDM and non-PTDM groups were compared regarding the numbers and days of antibiotic use. The numbers of antibiotics used (5.24±2.03 vs 4.31±1.68,
We classified patients who received treatment with a specific antibiotic during hospitalization after RT in the same category. The results showed that the incidence of PTDM in patients receiving carbapenems (P<0.050) was significantly higher than in those not receiving carbapenems (Table 3). In the specific antibiotics, the incidence of PTDM was significantly higher in patients receiving imipenem (P=0.015) and levofloxacin (P<0.001) than in those not receiving these antibiotics. In addition, no significant difference in the incidence of PTDM was observed between patients with and without use of other antibiotics (Supplementary Table 1).
UNIVARIATE AND MULTIVARIATE ANALYSIS:
Age, BMI, TAC C0 at 7, 14, and 28 days, TAC C0/D at 7 days, numbers and days of antibiotic use, imipenem, and levofloxacin were identified as being statistically significant in univariate logistic regression analysis (Table 4). The multivariate logistic regression model included all variables that were retained in the univariate analysis (P<0.060). We observed that older recipients (OR 1.049, 95% CI 1.009–1.086, P=0.014) and those with higher BMI (OR 1.178, 95% CI 1.046–1.325, P=0.007) after RT were significantly more likely to develop PTDM. Likewise, during follow-up, higher TAC C0/D at 7 days after RT (OR 1.159, 95% CI 1.005–1.137, P=0.042) and higher TAC C0 at 28 days after RT (OR 1.094, 95% CI 1.003–1.193, P=0.042) contributed to an increased risk of PTDM. Higher TAC C0 at 7 and 14 days after RT showed a nonsignificant effect on the risk of developing PTDM (P>0.05). Noticeably, the use of levofloxacin was an independent risk factor for PTDM, and patients receiving levofloxacin (OR=5.975, 95% CI 1.845–19.345, P=0.003) had a higher risk of developing PTDM. However, numbers and days of antibiotic use, and imipenem were not significantly associated with the development of PTDM.
Discussion
The incidence of PTDM greatly varies among transplant centers, ranging from 2% to 50%, which is related to different screening methods, diagnostic criteria, observation times, and postoperative immunosuppressive regimens, as well as races and diets in different countries and regions [16]. In RT patients, PTDM usually occurs 3–6 months after surgery [17]. Most RT patients have stress hyperglycemia in the short term after surgery, which is mainly caused by using high doses of glucocorticoids. Blood glucose tends to decrease with the decrease in glucocorticoid dose. Meanwhile, surgical stress response and post-transplant-related infections can lead to postoperative transient hyperglycemia. To avoid transient post-transplantation hyperglycemia, the time of PTDM determination in this study was from 1.5 months to 1 year after surgery [18].
Age and BMI have been identified as important risk factors for PTDM. The decline in pancreatic β cell function was caused by aging. It has been reported that the risk of PTDM increases significantly in RT patients older than 40 years, and the incidence of PTDM increases by 29% for every 10-year age increment [19,20]. Among the 37 patients with PTDM included in our study, 67.6% of patients were over 40 years old. It has been confirmed that overweight (BMI>25 kg/m2) is a risk factor for PTDM [21]. Another study found a 3.46-fold increased risk of PTDM when BMI was greater than 25 kg/m2 [22]. Consistently, high BMI in our study was associated with an increased risk of PTDM. Obesity can lead to insulin resistance in islet β cells and a reduced rate of glucose clearance, which can lead to PTDM [20].
Immunosuppressants are an important risk factor for PTDM, and approximately 20–30% of immunosuppressant therapy patients develop PTDM [23,24]. The triple immunosuppressive regimen of calcineurin inhibitor, mycophenolate mofetil, and glucocorticoid is commonly used. As a first-line calcineurin inhibitor, TAC has a greater potential to induce PTDM than cyclosporine [25]. The mechanism of TAC-induced PTDM has not been fully clarified. It was thought that TAC has direct toxic effects on pancreatic β cells and induces a reversible suppression of insulin secretion at the level of insulin mRNA transcription. The aggravation of insulin resistance by TAC was also a potential mechanism [26]. PTDM is associated with TAC blood concentrations. It has been reported that the risk of PTDM was significantly increased in patients with an initial TAC concentration >20 ng/ml after transplantation, and more than 80% of PTDM patients had an initial TAC concentration >20 ng/ml [27]. Another study found that TAC concentration >10 ng/ml in the first 3 months after transplantation was an important risk factor for PTDM [6]. In our study, the TAC C0 and C0/D of PTDM patients were generally higher than those of non-PTDM patients within 1 month after RT. The TAC C0/D at 7 days and TAC C0 at 28 days after RT had a significant positive correlation with the occurrence of PTDM.
The
Due to complex surgery and the weakening of the body’s immune defense by immunosuppressive agents, organ transplant patients have a high risk of infection. Therefore, the correct and reasonable application of antibiotics in the perioperative period plays an important role in reducing surgical site infection [29]. The regimen for perioperative antibiotics is different for each transplant center and its prophylaxis protocol is optimized based on local drug resistance patterns and the infection status of the donor and recipient. Cheng et al presented the characteristics of perioperative infection and commonly used antibiotics in their RT center. Cefoperazone-sulbactam was the most commonly prescribed antibiotic regimen, followed by piperacillin-tazobactam, carbapenems, linezolid, vancomycin, and polymyxin B, most of which are prophylactic medications [30]. In our transplant center, piperacillin-tazobactam and imipenem-cilastatin are the most commonly used drugs to prevent gram-negative bacilli in the perioperative period, and linezolid is the most commonly used to prevent gram-positive bacteria. Regarding the indication for usage of antibiotic, we found that piperacillin-tazobactam sodium, penicillin, imipenem, imipenem/cilastatin sodium, and linezolid are often used in combination to treat urinary tract infections, levofloxacin, amoxicillin/sulbactam sodium, cefixime, and other cephalosporin antibiotics are often used in combination to treat wound infections, and penicillin, piperacillin-tazobactam sodium, and moxifloxacin are often used in combination to treat lung infections.
The relationship between antibiotics and diabetes has gradually attracted increased attention. A retrospective cohort study found that repeated antibiotic exposure, including cephalosporin, macrolide, penicillin, and sulfonamide, increased the risk of diabetes [8]. Moreover, the risk of type I and type II diabetes increases with the duration and number of antibiotics [31]. Another Korean cohort study showed that the effect of antibiotics on the incidence of diabetes was dose-dependent, and people using multiple antibiotics had a higher risk of diabetes than those receiving a single antibiotic [32]. These studiessuggest a strong association between antibiotic exposure and diabetes. As a special type of diabetes mellitus, no study has reported the effect of antibiotics on the occurrence of PTDM. Therefore, we analyzed the correlation between postoperative antibiotics commonly used and PTDM in RT patients in our center and found that the incidence of PTDM was significantly higher in patients receiving quinolones than in patients without PTDM. We further analyzed the association between the use of specific antibiotics and PTDM. Through binary logistic regression, we found that levofloxacin (OR=5.975, 95% CI 1.845–19.345,
At present, fluoroquinolone drugs affecting PTDM have not been reported, but several studies have shown that fluoroquinolone antibiotics can cause hypo- or hyperglycemia in certain patients, and some possible mechanisms have been proposed. Ghaly et al showed that ciprofloxacin and moxifloxacin blocked ATP-dependent K+ channels and generated an increase in insulin release, and that the hypoglycemiceffect was dose-dependent [9]. However, Telfer et al found that fluoroquinolones were risk factors for type 2 diabetes, possibly because fluoroquinolones induce an intracellular magnesium deficit that can lead to insulin resistance [33]. Meanwhile, it has been suggested that there is a link between low magnesium levels and an elevated risk of post-transplant diabetes mellitus (PTDM) [34]. In our study, we performed a statistical analysis of magnesium levels before and 2 months after transplantation, but the results showed no significant difference in magnesium levels between patients with PTDM and those with non-PTDM. In addition, the use of imipenem was significantly associated with PTDM in univariate logistic regression (OR=2.386, 95% CI 1.204–4.730,
Our study has several limitations. This was a single-center retrospective study, and the number of patients included was not sufficient. The result may have been affected by the inclusion and exclusion criteria, which may have led to selection bias. In addition, RT patients receive multiple antibiotics, and it is difficult to evaluate the effect of multiple antibiotic combination therapy on the development of PTDM.
Conclusions
Our findings suggested that age, BMI, TAC C0, and C0/D after RT were associated with an increased risk of PTDM. Furthermore, antibiotics seem to be a potentially novel factor affecting PTDM.
Figures
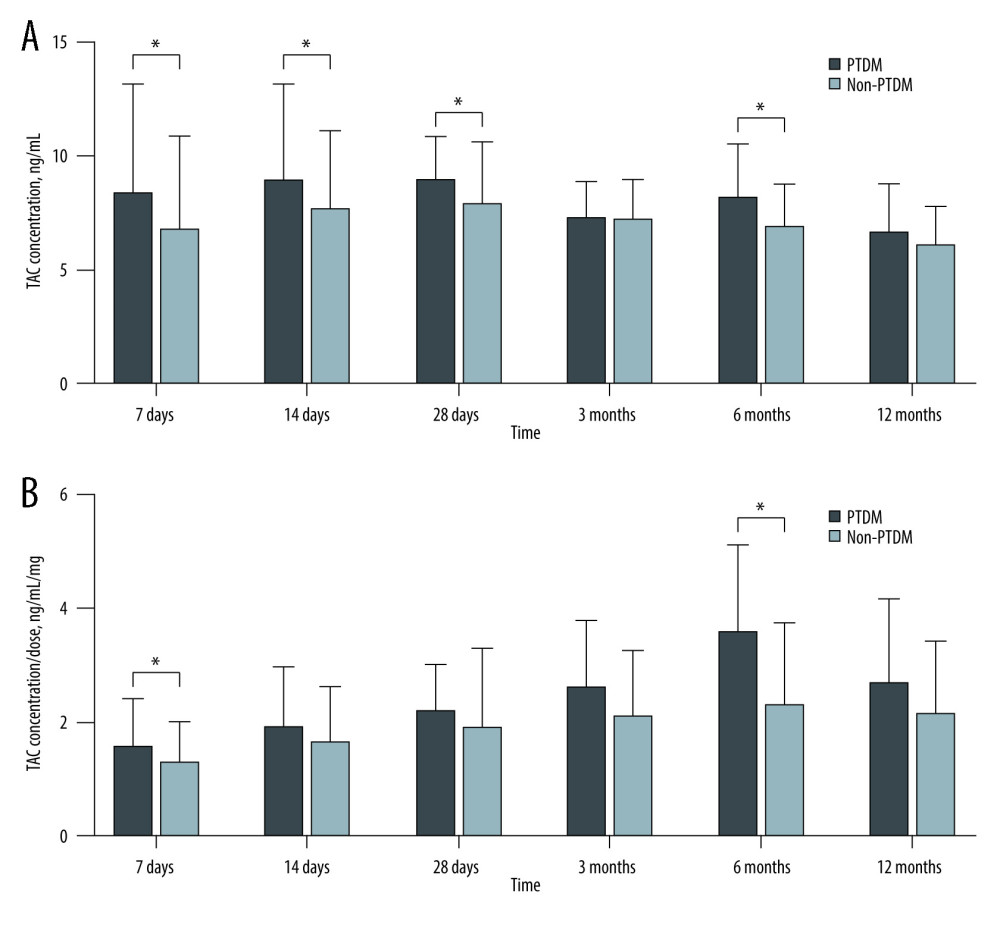 Figure 1. Comparison of TAC C0 and C0/D at different time points after transplantation between PTDM patients and non-PTDM patients(A) TAC C0 during 1 year of follow-up; (B) TAC C0/D ratio during 1 year of follow-up. Values are expressed as the mean±standard deviation. Quantitative variables were analyzed using the t test.
Figure 1. Comparison of TAC C0 and C0/D at different time points after transplantation between PTDM patients and non-PTDM patients(A) TAC C0 during 1 year of follow-up; (B) TAC C0/D ratio during 1 year of follow-up. Values are expressed as the mean±standard deviation. Quantitative variables were analyzed using the t test. 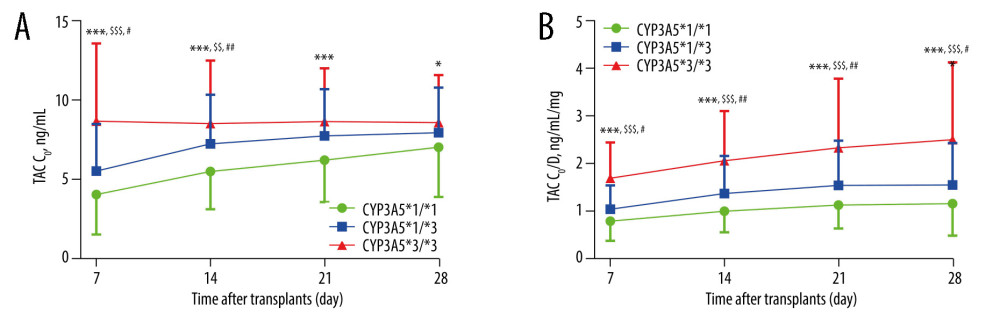 Figure 2. Influence of CYP3A5*3 polymorphism on TAC C0 and C0/DTAC C0: TAC trough level; TAC C0/D: TAC dose-adjusted trough level. (A, B) TAC C0 and C0/D over time by CYP3A5*3 genotype. genotype wild-type vs mutant homozygous genotype *: * P<0.05, ** P<0.01, *** P<0.001; wild-type vs mutant heterozygous genotype#: # P<0.05, ## P<0.01, ### P<0.001; mutant heterozygous genotype vs mutant homozygous genotype$: $ P<0.05, $$ P<0.01, $$$ P<0.001. Values are expressed as the mean±standard deviation. The data were analyzed by one-way ANOVA, with post hoc Bonferroni’s or Games-Howell’s multiple comparison tests based on homogeneity of variance.
Figure 2. Influence of CYP3A5*3 polymorphism on TAC C0 and C0/DTAC C0: TAC trough level; TAC C0/D: TAC dose-adjusted trough level. (A, B) TAC C0 and C0/D over time by CYP3A5*3 genotype. genotype wild-type vs mutant homozygous genotype *: * P<0.05, ** P<0.01, *** P<0.001; wild-type vs mutant heterozygous genotype#: # P<0.05, ## P<0.01, ### P<0.001; mutant heterozygous genotype vs mutant homozygous genotype$: $ P<0.05, $$ P<0.01, $$$ P<0.001. Values are expressed as the mean±standard deviation. The data were analyzed by one-way ANOVA, with post hoc Bonferroni’s or Games-Howell’s multiple comparison tests based on homogeneity of variance. Tables
Table 1. Clinical characteristics of renal transplant patients before transplantation.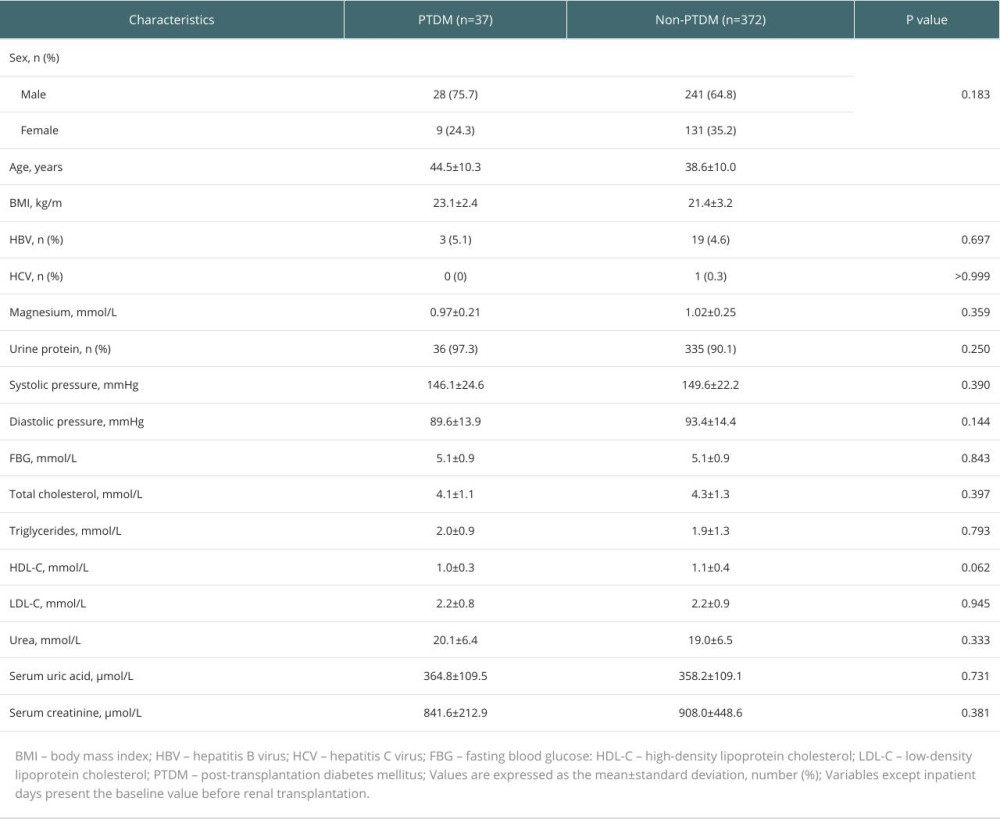 Table 2. Influence of CYP3A5*3, POR*28, ABCB1 (3435 C>T), and ABCB1 (1236 C>T) polymorphisms on PTDM.
Table 2. Influence of CYP3A5*3, POR*28, ABCB1 (3435 C>T), and ABCB1 (1236 C>T) polymorphisms on PTDM.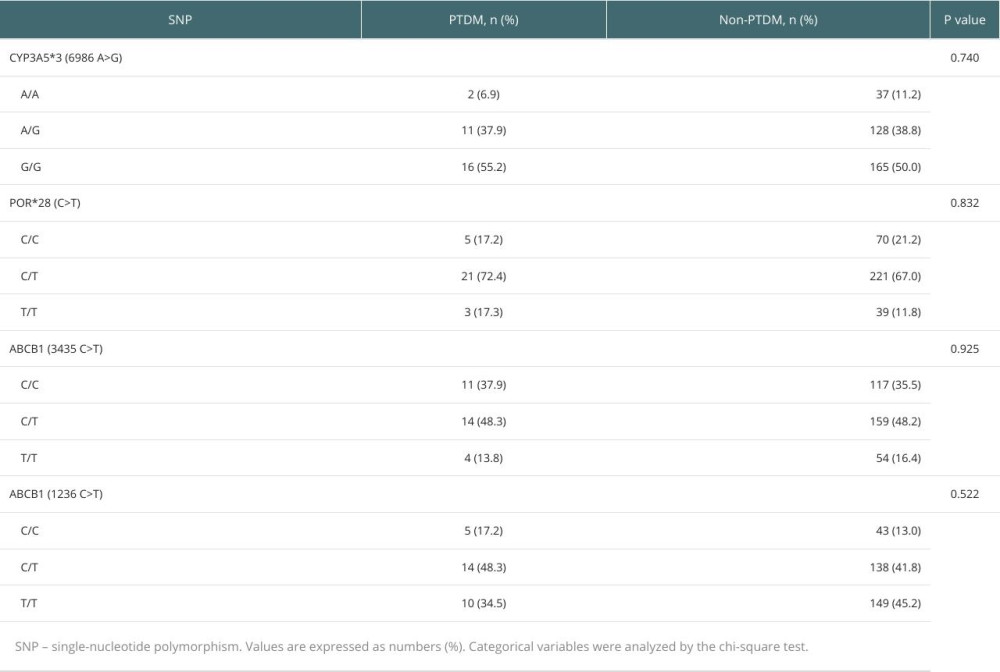 Table 3. Summary of antibiotic use in patients after renal transplantation.
Table 3. Summary of antibiotic use in patients after renal transplantation.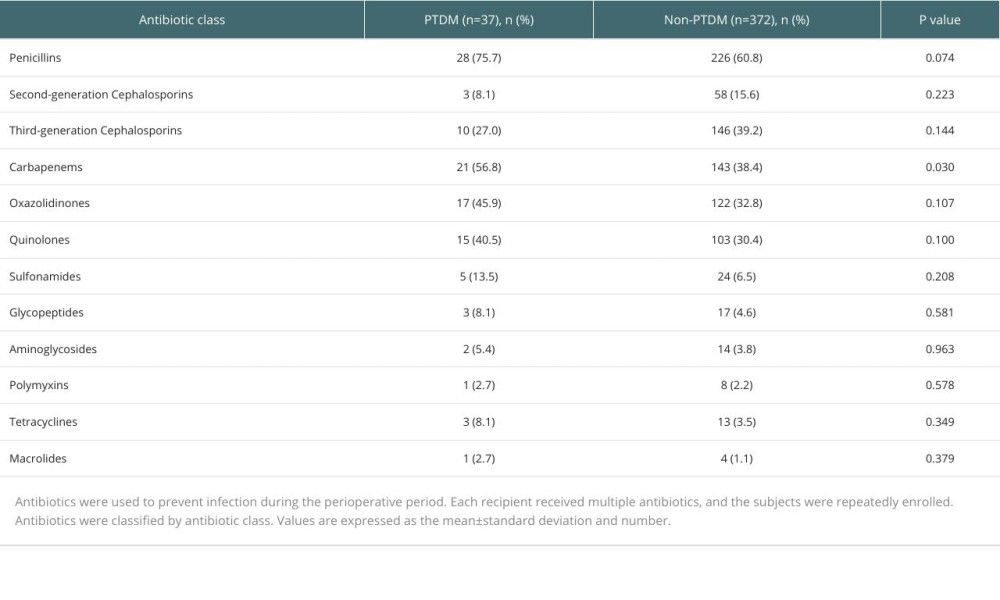 Table 4. Logistic regression analysis of risk factors for PTDM.
Table 4. Logistic regression analysis of risk factors for PTDM.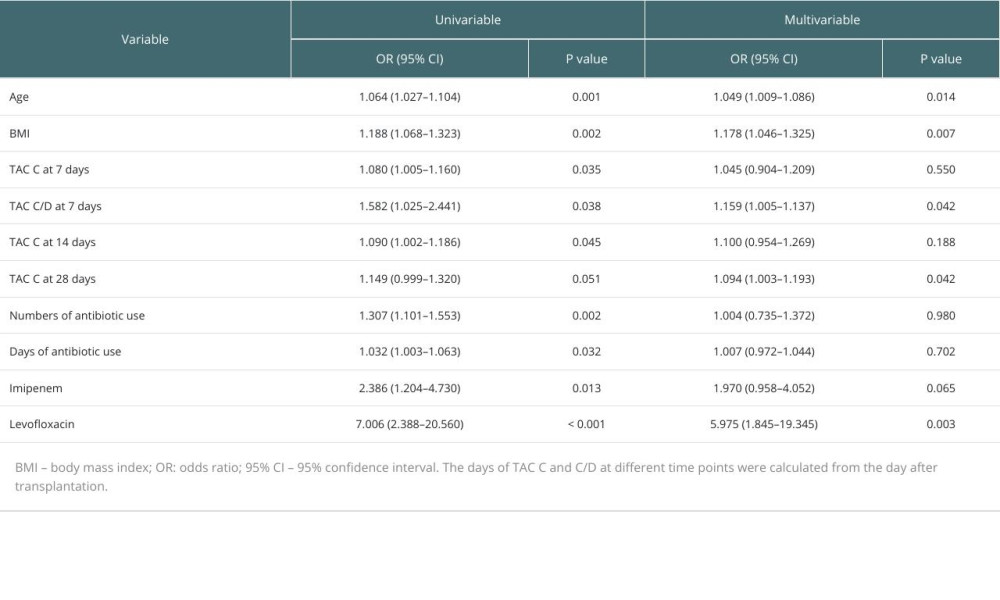 Supplementary Table 1. Information on patient antibiotic use during hospitalization and differences in particular antibiotic use between PTDM patients and non-PTDM patients after renal transplantation (n=409).
Supplementary Table 1. Information on patient antibiotic use during hospitalization and differences in particular antibiotic use between PTDM patients and non-PTDM patients after renal transplantation (n=409).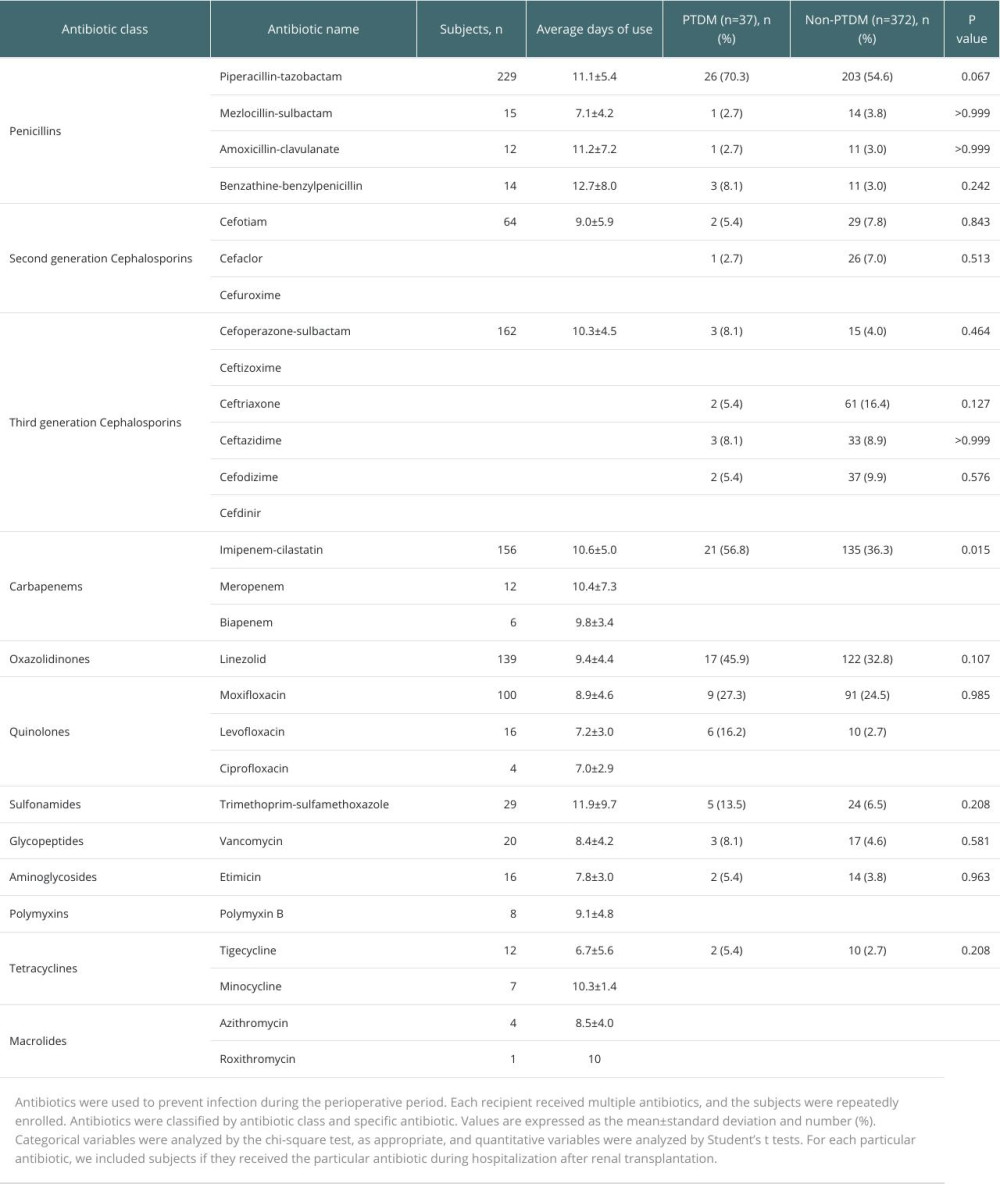
References
1. Ahmed SH, Biddle K, Augustine T, Azmi S, Post-transplantation diabetes mellitus: Diabetes Ther, 2020; 11(4); 779-801
2. Maskey R, New-onset diabetes after transplant (NODAT): Kathmandu Univ Med J (KUMJ), 2014; 12(48); 301-5
3. Jouve T, Noble J, Rostaing L, Malvezzi P, Tailoring tacrolimus therapy in kidney transplantation: Expert Rev Clin Pharmacol, 2018; 11(6); 581-88
4. Yu M, Liu M, Zhang W, Ming Y, Pharmacokinetics, pharmacodynamics and pharmacogenetics of tacrolimus in kidney transplantation: Curr Drug Metab, 2018; 19(6); 513-22
5. Agrawal V, Huang N, Miller WL, Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19: Pharmacogenet Genomics, 2008; 18(7); 569-76
6. Ajabnoor A, Nasser M, Khan N, Habhab W, Evaluation of tacrolimus trough level in patients who developed post-transplant diabetes mellitus after kidney transplantation: A retrospective single-center study in Saudi Arabia: Transplant Proc, 2020; 52(10); 3160-67
7. Kinn PM, Ince D, Outpatient and peri-operative antibiotic stewardship in solid organ transplantation: Transpl Infect Dis, 2022; 24(5); e13922
8. Davis PJ, Liu M, Alemi F, Prior antibiotic exposure and risk of type 2 diabetes among Veterans: Primary Care Diabetes, 2019; 13(1); 49-56
9. Granados J, Ceballos M, Amariles PHypo or hyperglycemia associated with fluoroquinolone use: Rev Med Chil, 2018; 146(5); 618-26 [in Spanish]
10. Yuan J, Hu YJ, Zheng J, Long-term use of antibiotics and risk of type 2 diabetes in women: A prospective cohort study: Int J Epidemiol, 2020; 49(5); 1572-81
11. Han Y, Jiang X, Ling Q, Antibiotics-mediated intestinal microbiome perturbation aggravates tacrolimus-induced glucose disorders in mice: Front Med, 2019; 13(4); 471-81
12. Fu L, Qiu Y, Shen L, The delayed effects of antibiotics in type 2 diabetes, friend or foe?: J Endocrinol, 2018; 238(2); 137-49
13. Sharif A, Hecking M, de Vries AP, Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions: Am J Transplant, 2014; 14(9); 1992-2000
14. Ling J, Dong LL, Yang XP, Effects of CYP3A5, ABCB1 and POR*28 polymorphisms on pharmacokinetics of tacrolimus in the early period after renal transplantation: Xenobiotica, 2020; 50(12); 1501-9
15. Cheng F, Li Q, Wang J, Hu M, Genetic polymorphisms affecting tacrolimus metabolism and the relationship to post-transplant outcomes in kidney transplant recipients: Pharmgenomics Pers Med, 2021; 14; 1463-74
16. Montori VM, Basu A, Erwin PJ, Posttransplantation diabetes: A systematic review of the literature: Diabetes Care, 2002; 25(3); 583-92
17. Cotovio P, Neves M, Rodrigues L, New-onset diabetes after transplantation: Assessment of risk factors and clinical outcomes: Transplant Proc, 2013; 45(3); 1079-83
18. Dos Santos Q, Hornum M, Terrones-Campos C, Posttransplantation diabetes mellitus among solid organ recipients in a Danish cohort: Transpl Int, 2022; 35; 10352
19. Malik RF, Jia Y, Mansour SG, Post-transplant diabetes mellitus in kidney transplant recipients: A multicenter study: Kidney360, 2021; 2(8); 1296-307
20. Siraj ES, Abacan C, Chinnappa P, Risk factors and outcomes associated with posttransplant diabetes mellitus in kidney transplant recipients: Transplant Proc, 2010; 42(5); 1685-89
21. Xu Y, Liang JX, Liu B, Prevalence and long-term glucose metabolism evolution of post-transplant diabetes mellitus in Chinese renal recipients: Diabetes Res Clin Pract, 2011; 92(1); 11-18
22. Shah T, Kasravi A, Huang E, Risk factors for development of new-onset diabetes mellitus after kidney transplantation: Transplantation, 2006; 82(12); 1673-76
23. Ghisdal L, Van Laecke S, Abramowicz MJ, New-onset diabetes after renal transplantation: risk assessment and management: Diabetes Care, 2012; 35(1); 181-88
24. Vincenti F, Friman S, Scheuermann E, Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus: Am J Transplant, 2007; 7(6); 1506-14
25. Penfornis A, Kury-Paulin S, Immunosuppressive drug-induced diabetes: Diabetes Metab, 2006; 32(5 Pt 2); 539-46
26. Bhat M, Pasini E, Das A, Diabetogenic effects of immunosuppression: An integrative analysis: Transplantation, 2020; 104(1); 211-21
27. Rodrigo E, Piñera C, de Cos MA, Evolution of tacrolimus blood levels and concentration-dose ratios in patients who develop new onset diabetes mellitus after kidney transplantation: Transpl Int, 2005; 18(10); 1152-57
28. Lancia P, Adam de Beaumais T, Elie V, Pharmacogenetics of post-transplant diabetes mellitus in children with renal transplantation treated with tacrolimus: Pediatr Nephrol, 2018; 33(6); 1045-55
29. Chan S, Ng S, Chan HP, Perioperative antibiotics for preventing post-surgical site infections in solid organ transplant recipients: Cochrane Database Syst Rev, 2020; 8(8); CD013209
30. Cheng F, Li Q, Wang J, Retrospective analysis of the risk factors of perioperative bacterial infection and correlation with clinical prognosis in kidney transplant recipients: Infect Drug Resist, 2022; 15; 2271-86
31. Boursi B, Mamtani R, Haynes K, Yang YX, The effect of past antibiotic exposure on diabetes risk: Eur J Endocrinol, 2015; 172(6); 639-48
32. Park SJ, Park YJ, Chang J, Association between antibiotics use and diabetes incidence in a nationally representative retrospective cohort among Koreans: Sci Rep, 2021; 11(1); 21681
33. Telfer SJ, Fluoroquinolone antibiotics and type 2 diabetes mellitus: Med Hypotheses, 2014; 83(3); 263-69
34. Cheungpasitporn W, Thongprayoon C, Harindhanavudhi T, Hypomagnesemia linked to new-onset diabetes mellitus after kidney transplantation: A systematic review and meta-analysis: Endocr Res, 2016; 41(2); 142-47
Figures
 Figure 1. Comparison of TAC C0 and C0/D at different time points after transplantation between PTDM patients and non-PTDM patients(A) TAC C0 during 1 year of follow-up; (B) TAC C0/D ratio during 1 year of follow-up. Values are expressed as the mean±standard deviation. Quantitative variables were analyzed using the t test.
Figure 1. Comparison of TAC C0 and C0/D at different time points after transplantation between PTDM patients and non-PTDM patients(A) TAC C0 during 1 year of follow-up; (B) TAC C0/D ratio during 1 year of follow-up. Values are expressed as the mean±standard deviation. Quantitative variables were analyzed using the t test. Figure 2. Influence of CYP3A5*3 polymorphism on TAC C0 and C0/DTAC C0: TAC trough level; TAC C0/D: TAC dose-adjusted trough level. (A, B) TAC C0 and C0/D over time by CYP3A5*3 genotype. genotype wild-type vs mutant homozygous genotype *: * P<0.05, ** P<0.01, *** P<0.001; wild-type vs mutant heterozygous genotype#: # P<0.05, ## P<0.01, ### P<0.001; mutant heterozygous genotype vs mutant homozygous genotype$: $ P<0.05, $$ P<0.01, $$$ P<0.001. Values are expressed as the mean±standard deviation. The data were analyzed by one-way ANOVA, with post hoc Bonferroni’s or Games-Howell’s multiple comparison tests based on homogeneity of variance.
Figure 2. Influence of CYP3A5*3 polymorphism on TAC C0 and C0/DTAC C0: TAC trough level; TAC C0/D: TAC dose-adjusted trough level. (A, B) TAC C0 and C0/D over time by CYP3A5*3 genotype. genotype wild-type vs mutant homozygous genotype *: * P<0.05, ** P<0.01, *** P<0.001; wild-type vs mutant heterozygous genotype#: # P<0.05, ## P<0.01, ### P<0.001; mutant heterozygous genotype vs mutant homozygous genotype$: $ P<0.05, $$ P<0.01, $$$ P<0.001. Values are expressed as the mean±standard deviation. The data were analyzed by one-way ANOVA, with post hoc Bonferroni’s or Games-Howell’s multiple comparison tests based on homogeneity of variance. Tables
 Table 1. Clinical characteristics of renal transplant patients before transplantation.
Table 1. Clinical characteristics of renal transplant patients before transplantation. Table 2. Influence of CYP3A5*3, POR*28, ABCB1 (3435 C>T), and ABCB1 (1236 C>T) polymorphisms on PTDM.
Table 2. Influence of CYP3A5*3, POR*28, ABCB1 (3435 C>T), and ABCB1 (1236 C>T) polymorphisms on PTDM. Table 3. Summary of antibiotic use in patients after renal transplantation.
Table 3. Summary of antibiotic use in patients after renal transplantation. Table 4. Logistic regression analysis of risk factors for PTDM.
Table 4. Logistic regression analysis of risk factors for PTDM. Table 1. Clinical characteristics of renal transplant patients before transplantation.
Table 1. Clinical characteristics of renal transplant patients before transplantation. Table 2. Influence of CYP3A5*3, POR*28, ABCB1 (3435 C>T), and ABCB1 (1236 C>T) polymorphisms on PTDM.
Table 2. Influence of CYP3A5*3, POR*28, ABCB1 (3435 C>T), and ABCB1 (1236 C>T) polymorphisms on PTDM. Table 3. Summary of antibiotic use in patients after renal transplantation.
Table 3. Summary of antibiotic use in patients after renal transplantation. Table 4. Logistic regression analysis of risk factors for PTDM.
Table 4. Logistic regression analysis of risk factors for PTDM. Supplementary Table 1. Information on patient antibiotic use during hospitalization and differences in particular antibiotic use between PTDM patients and non-PTDM patients after renal transplantation (n=409).
Supplementary Table 1. Information on patient antibiotic use during hospitalization and differences in particular antibiotic use between PTDM patients and non-PTDM patients after renal transplantation (n=409). In Press
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
03 Apr 2024 : Review article
Alternative Therapies in Transplantology as a Promising Perspective in MedicineAnn Transplant In Press; DOI: 10.12659/AOT.943387
15 Apr 2024 : Original article
Physical Activity Levels in Transplant RecipientsAnn Transplant In Press; DOI: 10.12659/AOT.944101
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








